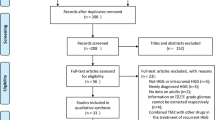
Abstract
Purpose
Brain radiotherapy combined with concomitant and six cycles of adjuvant temozolomide (TMZ) is the standard treatment for newly diagnosed high-grade gliomas (HGGs). However, the optimal number of cycles of TMZ is still controversial. We conducted this retrospective cohort study to evaluate whether prolonging adjuvant TMZ beyond six cycles resulted in better survival outcomes.
Methods
Patients with high-grade gliomas treated with standard brain radiotherapy combined with TMZ were retrospectively analysed. The duration of adjuvant TMZ ranged from 6 to 12 cycles. Those with 6 cycles of adjuvant TMZ were defined as the standard STUPP group, and those with 7–12 cycles were called the extended STUPP group. Median progression-free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method. The Cox proportional hazards model was adopted to estimate the Hazard ratio (HR) associated with PFS and OS.
Results
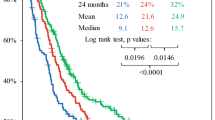
From September 2011 to May 2021, 372 patients were eligible (143 in the standard group, 229 in the extended group). Patients who received extended STUPP had better PFS and OS compared with standard STUPP. The median PFS for the standard STUPP group was 12 months and for the extended STUPP group 22 months (log-rank P < 0.001). The median OS for the standard STUPP group and extended STUPP group were 12 months and 36 months, respectively (log-rank P < 0.001). In the subgroup analysis, the two treatments did not differ in IDH-mutated patients, while patients with IDH wild-type had a significantly better response to extended treatment than to standard treatment (PFS: log-rank P = 0.004; OS log-rank P = 0.001). Patients with MGMT promoter methylation treated with extended STUPP obtained longer PFS and OS than those treated with standard STUPP (PFS: log-rank P = 0.015; OS log-rank P = 0.010). Adverse events including leukopenia (P < 0.001), thrombocytopenia (P = 0.090), fatigue (P < 0.001) and nausea/vomiting (P = 0.004) were more frequent in the extended group.
Conclusion
Extended TMZ treatment was superior to standard 6-cycle TMZ for both PFS and OS. The incidence of toxicities in extended group was higher but tolerable.






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sanai N, Alvarez-Buylla A, Berger MS (2005) Neural stem cells and the origin of gliomas. N Engl J Med 353(8):811–822
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Balañá C, Vaz MA, Lopez D, de la Peñas R, García-Bueno JM, Molina-Garrido MJ, Sepúlveda JM, Cano JM, Bugés C, Sanz SM et al (2014) Should we continue temozolomide beyond six cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin translational oncology: official publication Federation Span Oncol Soc Natl Cancer Inst Mexico 16(3):273–279
Roldan Urgoiti GB, Singh AD, Easaw JC (2012) Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol 108(1):173–177
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K et al (2017) Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 318(23):2306–2316
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31(32):4085–4091
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bahr O et al (2020) Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 6(7):1003–1010
Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, Nghiemphu L, Kaley T, Colman H, McCluskey C et al (2021) Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin Cancer Res 27(4):1048–1057
Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M, Felsberg J, Beyer U, Loffler-Wirth H, Kaulich K et al (2015) Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129(5):679–693
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM et al (2016) Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 164(3):550–563
Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, Shimamura T, Niida A, Motomura K, Ohka F et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47(5):458–468
Blumenthal DT, Gorlia T, Gilbert MR, Kim MM, Burt Nabors L, Mason WP, Hegi ME, Zhang P, Golfinopoulos V, Perry JR et al (2017) Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol 19(8):1119–1126
Balana C, Vaz MA, Manuel Sepulveda J, Mesia C, Del Barco S, Pineda E, Munoz-Langa J, Estival A, de Las Penas R, Fuster J et al (2020) A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14 – 01). Neuro Oncol 22(12):1851–1861
Hau P, Koch D, Hundsberger T, Marg E, Bauer B, Rudolph R, Rauch M, Brenner A, Rieckmann P, Schuth J et al (2007) Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology 68(9):688–690
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251
Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A et al (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981 – 22981/CE.3. Lancet Oncol 9(1):29–38
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003
Everhard S, Kaloshi G, Crinière E, Benouaich-Amiel A, Lejeune J, Marie Y, Sanson M, Kujas M, Mokhtari K, Hoang-Xuan K et al (2006) MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol 60(6):740–743
Vinagre J, Pinto V, Celestino R, Reis M, Populo H, Boaventura P, Melo M, Catarino T, Lima J, Lopes JM et al (2014) Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch 465(2):119–133
Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N et al (2013) Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 126(2):267–276
Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H (2013) TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol 126(6):931–937
Lotsch D, Ghanim B, Laaber M, Wurm G, Weis S, Lenz S, Webersinke G, Pichler J, Berger W, Spiegl-Kreinecker S (2013) Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro Oncol 15(4):423–432
Acknowledgements
We acknowledge Dr. Ye Hong of Sun Yat-sen University Cancer Center for her valuable advice on the data analysis in our study.
Funding
The study was supported by National Natural Science Foundation of China (Grant Nos. 82172842, and 81803104 and 81672386), the Sichuan Province Science and Technology Support Program (Grant Nos. 2021YFSY008, 2020YFS0276), West China Nursing Discipline Development Special Fund Project (Grant No. HXHL21008), the Technology Innovation Project of Chengdu Science and Technology Bureau (Grant No. 2019-YF05-00459-SN), Postdoctoral research and Development Fund and Translational medicine fund of West China Hospital (Grant Nos. 2020HXBH119 and CGZH19002), National Key R&D Program of China (2018YFA0108604), the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (21HXFH046), the project of Sichuan Science and Technology Bureau (22ZDYF0798), and Clinical Incubation Program of West China Hospital, SCU(2018HXFU008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Xingchen Peng, Ping Ai, and Jingjing Wang contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jingjing Wang, Yan Huang, Feng Zhao, Jianhui Chen, Ling He, Zheran Liu and Yiyan Pei. The first draft of the manuscript was written by Jingjing Wang and Yan Huang. Xingchen Peng and Ping Ai reviewed the manuscript and provided intellectual contributions. Jingjing Wang, Yan Huang, Zhigong Wei, and Ruidan Li edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University.
Consent to participate
The Ethics Committee on Biomedical Research, West China Hospital of Sichuan University agreed to exempt the informed consent form for this study.
Consent to publish
Our manuscript did not contain any individual person’s data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingjing Wang and Yan Huang contributed equally to this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Huang, Y., Zhao, F. et al. Standard or extended STUPP? Optimal duration of temozolomide for patients with high-grade gliomas: a retrospective analysis. J Neurooncol 160, 433–443 (2022). https://doi.org/10.1007/s11060-022-04162-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04162-w