Abstract
Introduction
The management of skull base malignancies continues to evolve with improvements in surgical technique, advances in radiation delivery and novel systemic agents.
Methods
In this review, we aim to discuss in detail the management of common skull base pathologies which typically require multimodality therapy, focusing on the radiotherapeutic aspects of care.
Results
Technological advances in the administration of radiation therapy have led to a wide variety of different treatment strategies for the treatment of skull base malignances, with outcomes summarized herein.
Conclusion
Radiation treatment plays a key and critical role in the management of patients with skull base tumors. Recent advancements continue to improve the risk/benefit ratio for radiotherapy in this setting.

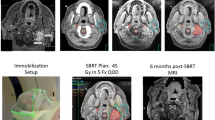
(modified from Wang et al. [1])
Similar content being viewed by others
Abbreviations
- EOR:
-
Extent of resection
- GTR:
-
Gross total resection
- NTR:
-
Near total resection
- STR:
-
Subtotal resection
- PR:
-
Partial resection
- PFS:
-
Progression free survival
- OS:
-
Overall survival
- LC:
-
Local control
- LRFS:
-
Local recurrence free survival
- TSS:
-
Tumor specific survival
- yo:
-
Years old
- NS:
-
Not specified
- (N)SBM:
-
(Non-) skull base meningioma
- ChS:
-
Chondrosarcoma
- ChD:
-
Chordoma
- DI:
-
Diabetes insipidus
- QOL:
-
Quality of life
- ADL:
-
Activity of daily living
- RR:
-
Retrospective review
- SR:
-
Systematic review
- RT:
-
Radiation therapy
- (h)FSRT:
-
(Hypo)fractionated stereotactic radiosurgery
- SRS:
-
Stereotactic radiosurgery
- GKRT:
-
Gamma-knife radiation therapy
- IMRT:
-
Intensity modulated radiation therapy
References
Wang H, Yang JN, Zhang XD et al (2019) Treatment-plan comparision of three advanced radiation treatment modalities for fractionated stereotactic radiotherapy to the head and neck. Int J Med Phys 8:106–120
Sturgis EM, Potter BO (2003) Sarcomas of the head and neck region. Curr Opin Oncol 15:239–252
De Bree R, Van Der Waal I, De Bree E et al (2010) Management of adult soft tissue sarcomas of the head and neck. Oral Oncol 46:786–790
Wu AW, Suh JD, Metson R et al (2012) Prognostic factors in sinonasal sarcomas: analysis of the surveillance, epidemiology and end result database. Laryngoscope 122:2137–2142
Szablewski V, Neuville A, Terrier P et al (2015) Adult sinonasal soft tissue sarcoma: analysis of 48 cases from the French Sarcoma Group database. Laryngoscope 125:615–623
Pamir MN, Ozduman K (2006) Analysis of radiological features relative to histopathology in 42 skull-base chordomas and chondrosarcomas. Eur J Radiol 58:461–470
Welzel T, Meyerhof E, Uhl M et al (2018) Diagnostic accuracy of DW MR imaging in the differentiation of chordomas and chondrosarcomas of the skull base: A 3.0-T MRI study of 105 cases. Eur J Radiol 105:119–124
Li L, Wang K, Ma X et al (2019) Radiomic analysis of multiparametric magnetic resonance imaging for differentiating skull base chordoma and chondrosarcoma. Eur J Radiol 118:81–87
Santegoeds RGC, Temel Y, Beckervordersandforth JC et al (2018) State-of-the-art imaging in human chordoma of the skull base. Curr Radiol Rep 6(5):16
Weber DC, Malyapa R, Albertini F et al (2016) Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 120:169–174
Bakker SH, Jacobs WCH, Pondaag W et al (2018) Chordoma: a systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur Spine J 27:3043–3058
Amit M, Na'ara S, Binenbaum Y et al (2014) Treatment and outcome of patients with skull base chordoma: a meta-analysis. J Neurol Surg B 75:383–390
Di Maio S, Temkin N, Ramanathan D et al (2011) Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg 115:1094–1105
Bloch O, Parsa AT (2013) Skull base chondrosarcoma: evidence-based treatment paradigms. Neurosurg Clin N Am 24:89–96
Van Gompel JJ, Janus JR (2015) Chordoma and chondrosarcoma. Otolaryngol Clin N Am 48:501–514
Komotar RJ, Starke RM, Raper DM et al (2011) The endoscope-assisted ventral approach compared with open microscope-assisted surgery for clival chordomas. World Neurosurg 76:318–327
Di Maio S, Rostomily R, Sekhar LN (2012) Current surgical outcomes for cranial base chordomas: cohort study of 95 patients. Neurosurgery 70:1355–1360
Tzortzidis F, Elahi F, Wright DC et al (2006) Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chondrosarcomas. Neurosurgery 58:1090–1098
Stacchiotti S, Gronchi A, Fossati P et al (2017) Best practices for the management of local-regional recurrent chordoma: a position paper by the Chordoma Global Consensus Group. Ann Oncol 28:1230–1242
Stacchiotti S, Sommer J (2015) Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol 16:e71–83
Iyer A, Kano H, Kondziolka D et al (2013) Postsurgical management strategies in patients with skull base chondrosarcomas. CNS Oncol 2:203–208
Bloch OG, Jian BJ, Yang I et al (2009) A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci 16:1547–1551
Rosenberg AE, Nielsen GP, Keel SB et al (1999) Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol 23:1370–1378
Almefty K, Pravdenkova S, Colli BO et al (2007) Chordoma and chondrosarcoma: similar, but quite different, skull base tumors. Cancer 110:2457–2467
Raza SM, Gidley PW, Meis JM et al (2017) Multimodality treatment of skull base chondrosarcomas: the role of histology specific treatment protocols. Neurosurgery 81:520–530
Kano H, Niranjan A, Lunsford LD (2019) Radiosurgery for chordoma and chondrosarcoma. Prog Neurol Surg 34:207–214
Uhl M, Mattke M, Welzel T et al (2014) High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer 120:1579–1585
Feuvret L, Bracci S, Calugaru V et al (2016) Efficacy and safety of adjuvant proton therapy combined with surgery for chondrosarcoma of the skull base: a retrospective, population-based study. Int J Radiat Oncol Biol Phys 95:312–321
Lu VM, O'Connor KP, Mahajan A et al (2020) Carbon ion radiotherapy for skull base chordomas and chondrosarcomas: a systematic review and meta-analysis of local control, survival, and toxicity outcomes. J Neurooncol. https://doi.org/10.1007/s11060-020-03464-1
Ahmed SK, Brown PD, Foote RL (2018) Protons vs photons for brain and skull base tumors. Semin Radiat Oncol 28:97–107
McDonald MW, Linton OR, Moore MG et al (2016) Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int J Radiat Oncol Biol Phys 95:304–311
Raza SM, Bell D, Freeman JL et al (2018) Multimodality management of recurrent skull base chordomas: factors impacting tumor control and disease-specific survival. Oper Neurosurg (Hagerstown) 15:131–143
Alahmari M, Temel Y (2019) Skull base chordoma treated with proton therapy: a systematic review. Surg Neurol Int 10:96
Nikoghosyan AV, Karapanagiotou-Schenkel I, Munter MW et al (2010) Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer. 10:607
Munzenrider JE, Liebsch NJ (1999) Proton therapy for tumors of the skull base. Strahlenther Onkol 175:57–63
Uhl M, Mattke M, Welzel T et al (2014) Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 120:3410–3417
Mattke M, Vogt K, Bougatf N et al (2018) High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer 124:2036–2044
Frezza AM, Cesari M, Baumhoer D et al (2015) Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51:374–381
Italiano A, Mir O, Cioffi A et al (2013) Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol 24:2916–2922
Stacchiotti S, Longhi A, Ferraresi V et al (2012) Phase II study of imatinib in advanced chordoma. J Clin Oncol 30:914–920
Stacchiotti S, Morosi C, Lo Vullo S et al (2018) Imatinib and everolimus in patients with progressing advanced chordoma: a phase 2 clinical study. Cancer 124:4056–4063
Vujovic S, Henderson S, Presneau N et al (2006) Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 209:157–165
Gill CM, Fowkes M, Shrivastava RK (2019) Emerging therapeutic targets in chordomas: a review of the literature in the genomic era. Neurosurgery 86(2):E118–E123
Eilber FC, Dry SM (2008) Diagnosis and management of synovial sarcoma. J Surg Oncol 97(4):314–320
Palmerini E, Staals EL, Alberghini M et al (2009) Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer 115(13):2988–2998
Harb WJ, Luna MA, Patel SR et al (2007) Survival in patients with synovial sarcoma of the head and neck: association with tumor location, size, and extension. Head Neck 29(8):731–740
Al-Daraji W, Lasota J, Foss R et al (2009) Synovial sarcoma involving the head: analysis of 36 cases with predilection to the parotid and temporal regions. Am J Surg Pathol 33(10):1494–1503
Cihak RA, Lydiatt WM, Lydiatt DD et al (1997) Synovial sarcoma of the head and neck: chromosomal translocation (x;18) as a diagnostic aid. Head Neck 19:549–553
WHO (2020) WHO classification of soft tissue and bone tumors, vol 3, 5th edn. International Agency for Research on Cancer, Lyon
Mullen JR, Zagars GK (1994) Synovial sarcoma outcome following conservation surgery and radiotherapy. Radiother Oncol 33(1):23–30
Colville RJ, Charlton F, Kelly CG et al (2005) Multidisciplinary management of head and neck sarcomas. Head Neck 27:814–824
Shmookler BM, Enzinger FM, Brannon RB et al (1982) Orofacial synovial sarcoma: a clinicopathologic study of 11 new canses and review of literature. Cancer 50:269–276
Ji T, Ma CY, Ow A et al (2011) Synovial sarcoma involving skull base—a retrospective analysis of diagnosis and treatment of 21 cases in one institution. Oral Oncol 47:671–676
Lee N, Shin E (2008) Treatment outcomes for patients with synovial sarcoma of the head and neck. Expert Rev Anticancer Ther 8(3):371–373
Clair JM, Arshi A, Abemayor E et al (2016) Synovial cell sarcoma of the head and neck: an analysis of 167 cases using the SEER database. JAMA Otolaryngol Head Neck Surg 142(6):576–583
Siegal GP, Oliver WR, Reinus WR et al (1987) Primary Ewing’s sarcoma involving the bones of the head and neck. Cancer 60(11):2829–2840
Choi EY, Gardner JM, Lucas DR et al (2014) Ewing sarcoma. Semin Diagn Pathol 31(1):39–47
Desai KI, Nadkami TD, Goel A et al (2000) Primary Ewing’s sarcoma of the cranium. Neurosurgery 46(1):62–68
Womer RB, West DC, Krailo MD et al (2012) Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 30(33):4148–4154
Mascarenhas L, Felgenhauer JL, Bond MC et al (2016) Pilot study of adding vincristine, topotecan, and cyclophosphamide to interval compressed chemotherapy in newly diagnosed patients with localized Ewing sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 63(3):493–498
Biswas B, Thakar A, Mohanti BK et al (2015) Prognostic factors in head and neck Ewing sarcoma family to tumors. Laryngoscope 125:E112–E117
Olson MD, Van Abel KM, Wehrs RN et al (2018) Ewing sarcoma of the head and neck: the Mayo Clinic experience. Head Neck 40(9):1999–2006
Kharod SM, Indelicato DJ, Rotondo RL et al (2020) Outcomes following proton therapy for Ewing sarcoma of the cranium and skull base. Pediatr Blood Cancer 67(2):e28080
Patel AJ, Rao VY, Fox BD et al (2011) Radiation-induced osteosarcomas of the calvarium and skull base. Cancer 117:2120–2126
Wu G, Liang Q, Liu Y (2017) Primary osteosarcoma of the frontal bone. Medicine (Baltimore) 96(51):e9392
Caron AS, Hajdu SI, Strong EW (1971) Osteogenic sarcoma of the facial and cranial bones. A review of forty-three cases. Am J Surg 122:719–725
Guo Z, Hu K, Zhao B et al (2017) Osteosarcoma of the skull base: an analysis of 19 cases and literature review. J Clin Neurosci 44:133–142
Chen Y, Shen Q, Gokavarapu S et al (2016) Osteosarcoma of head and neck: a retrospective study on prognostic factors from a single institute database. Oral Oncol 58:1–7
Smith RB, Apostolakis LW, Karnell LH et al (2003) National cancer data base report on osteosarcoma of the head and neck. Cancer 98:1670–1680
Ciernik FI, Niemierko A, Harmon DC et al (2011) Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 117(19):4522–4530
DeLaney TF, Park L, Goldberg SI et al (2005) Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 61(2):492–498
Lewis JT, Oliveira AM, Nascimento AG et al (2012) Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol 36(4):517–525
Stelow EB, Bishop JA (2017) Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the nasal cavity, paranasal sinuses and skull base. Head and Neck Pathol 11:3–15
Carter CS, East EG, McHugh JB (2018) Biphenotypic sinonasal sarcoma: a review and update. Arch Pathol Lab Med 142(10):1196–1201
Rooper LM, Huang SC, Antonescu CR et al (2016) Biphenotypic sinonasal sarcoma: an expanded immunoprofile including consistent nuclear beta-catenin positivity and absence of SOX10 expression. Hum Pathol 55:44–50
Fritchie KJ, Jin L, Wang X et al (2016) Fusion gene profile of biphenotypic sinonasal sarcoma: an analysis of 44 cases. Histophatology 69(6):930–936
Miglani A, Lal D, Weindling SM et al (2019) Imaging characteristics and clinical outcomes of biphenotypic sinonasal sarcoma. Laryngoscope Investig Otolaryngol 4(5):484–488
Kuhn AL, Jalisi S, Nishin M et al (2019) Biphenotypic sinonasal sarcoma—description of radiologic, intraoperative and pathologic findings. Otolaryngol Case Rep 11:100113
Siddiqui F, Smith RV, Yom SS et al (2017) ACR appropriateness criteria nasal cavity and paranasal sinus cancers. Head Neck 39(3):407–418
Cannon RB, Wiggins RH III, Witt BL et al (2017) Imaging and outcomes for a new entity: low-grade sinonasal sarcoma with neural and myogenic features. J Neurol Surg Rep 78:e15–e19
Wagner E (1870) Das tuberkelahnliche lymphadenom (der cytogene oder reticulirte tuberkel). Arch Heilk (Leipig) 11:497
Gengler C, Guillou L (2006) Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 48:63–74
Schiariti M, Goetz P, El-Maghraby H et al (2011) Hemangiopeericytoma: long-term outcome revisited. J Neurosurg 114(3):747–755
Stout AP, Murray MR (1942) Hemangiopericytoma a vascular tumor featuring Zimmermann’s pericytes. Ann Surg 116(1):26–33
Fritchie KJ, Jin L, Rubin BP et al (2016) NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol 75(3):263–271
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Ghia AJ, Chang EL, Allen PK et al (2013) Intracranial hemangiopericytoma: patterns of failure and the role of radiation therapy. Neurosurgery 73(4):624–630
Sonabend AM, Zacharia BE, Goldstein H et al (2014) The role of adjuvant radiotherapy in the treatment of hemangiopericytoma: a surveillance, epidemiology, and end results analysis. J Neurosurg 120(2):300–308
Patel AR, Flores BC, Ban VS et al (2017) Intracranial hemangiopericytomas: recurrence, metastasis, and radiotherapy. J Neurol Surg B 78(4):324–330
Combs SE, Thilmann C, Debus J et al (2005) Precision radiotherpay for hemangiopericytomas of the central nervous system. Cancer 104(11):2457–2465
George S, Merriam P, Maki RG et al (2009) Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 27(19):3154–3160
Valentin T, Fournier C, Penel N et al (2013) Sorafenib in patients with progressive malignant solitary fibrous tumors: a subgroup analysis from a phase II study of the French Sarcoma Group (GSF/GETO). Investig New Drugs 31(6):1626–1627
Park MS, Patel SR, Ludwig JA et al (2011) Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangoipericytoma and malignant solitary fibrous tumor. Cancer 117(21):4939–4947
Batsakis JG, Rice DH, Solomon AR (1980) The pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg 2:497–508
Ayiomamitis A, Parker L, Havas T (1988) The epidemiology of malignant neoplasms of the nasal cavities, the paransal sinues and the middle ear in Canada. Arch Otorhinolaryngol 244:367–371
Turner JH, Reh DD (2012) Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 34(6):877–885
Abu-Ghanem S, Horowitz G, Abergel A et al (2015) Elective neck irradiation versus observation in squamous cell carcinoma of the maxillary sinus with N0 neck: a meta-analysis and review of the literature. Head Neck 37:1823–1828
Faisal M, Seemann R, Lill C et al (2020) Elective neck treatment in sinonasal undifferentiated carcinoma: systematic review and meta-analysis. Head Neck 42:1057–1066
Jiang W, Mohamed ASR, Fuller CD et al (2016) The role of elective nodal irradiation for esthesioneuroblastoma patients with clinically negative neck. Pract Radiat Oncol 6:241–247
Song X, Huang C, Wang S et al (2020) Neck management in patients with olfactory neuroblastoma. Oral Oncol 101:104505
Robin TP, Jones BL, Gordon OM et al (2017) A comprehensive analysis of treatment modalities for sinonasal malignancies. Cancer 123(16):3040–3049
Wiegner EA, Daly ME, Murphy JD et al (2012) Intensity-modulated radiotherapy for tumors of the nasal cavity and paranasal sinuses: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 83(1):243–251
Al-Mamgani A, Monserez D, Van Rooij P et al (2012) Highly-conformal intensity-modulated radiotherapy reduced toxicity without jeopardizing outcome in patients with paranasal sinus cancer treated by surgery and radiotherapy or (chemo)radiation. Oral Oncol 48:905–911
Toyomasu Y, Demizu Y, Matsuo Y et al (2018) Outcomes of patients with sinonasal squamous cell carcinoma treated with particle therapy using protons or carbon ions. Int J Radiat Oncol Biol Phys 5(1):1096–1103
Daly ME, Chen AM, Bucci KM et al (2007) Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 67(1):151–157
Fan M, Kang JJ, Lee A et al (2020) Outcomes and toxicities of definitive radiotherapy and reirradiation using 3-dimensional conformal or intensity-modulated (pencil beam) proton therapy for patients with nasal cavity and paranasal sinus malignancies. Cancer 126(9):1905–1916
Patel SH, Wang Z, Wong WW et al (2014) Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 15:1027–1038
Zenda S, Kohno R, Kawashima M et al (2011) Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 81(5):1473–1478
Russo AL, Adams JA, Weyman EA et al (2016) Long-term outcomes after proton beam therapy for sinonasal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 95(1):368–376
Dagan R, Bryant C, Li Z et al (2016) Outcomes of sinonasal cancer treated with proton therapy. Int J Radiat Oncol Biol Phys 95(1):377–385
Yu NY, Gamez ME, Hartsell WF et al (2019) A multi-institutional experience of proton beam therapy for sinonasal tumors. Adv Radiat Oncol 4(4):689–698
Barnes L (1986) Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinues. Am J Surg Pathol 10:192–202
Leivo I (2016) Sinonasal adenocarcinoma: update on classification, immunophenotype and molecular features. Head Neck Pathol 10(1):68–74
Thompson LDR, Franchi A (2018) New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Virchows Arch 472:315–330
Lund VJ, Chisholm EJ, Takes RP et al (2012) Evidence for treatment strategeis in sinonasal adenocarcinoma. Head Neck 34:1168–1178
Duprez F, Madani I, Morbee L et al (2012) IMRT for sinonasal tumors minimizes severe late ocular toxicity and preserves disease control and survival. Int J Radiat Oncol Biol Phys 83(1):252–259
Fordice J, Kershaw C, El-Naggar A et al (1999) Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg 125(2):149–152
Lupinetti AD, Roberts DB, Williams MD et al (2007) Sinonasal adenoid cystic carcinoma: the M.D. Anderson Cancer Center experience. Cancer 110(12):2726–2731
Bishop JA, Ogawa T, Stelow EB et al (2013) Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck restricted to the sinonasal tract. Am J Surg Pathol 37(6):836–844
Miller ED, Blakaj DM, Swanson BJ et al (2017) Sinonasal adenoid cystic carcinoma: treatment outcomes and association with human papillomavirus. Head Neck 39:1405–1411
Koto M, Demizu Y, Saitoh JI et al (2018) Definitive carbon-ion radiation therapy for locally advanced sinonasal malignant tumors: subgroup analysis of a multicenter study be the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS). Int J Radiat Oncol Biol Phys 102(2):353–361
Frierson HF Jr, Mills SE, Fechner RE et al (1986) Sinonasal undifferentiated carcinoma. An aggressive neoplasm deivered from Schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol 10(11):771–779
Levine PA, Frierson HF Jr, Stewart FM et al (1987) Sinonasal undifferentiated carcinoma: a distinctive andhighly aggressive neoplasm. Laryngoscope 97(8 Pt 1):905–908
Mendenhall WM, Mendenhall CM, Riggs CE Jr et al (2006) Sinonasal undifferentiated carcinoma. Am J Clin Oncol 29(1):27–31
Mourad WF, Hauerstock D, Shourbaji RA et al (2013) Trimodality management of sinonasal undifferentiated carcinoma and review of the literature. Am J Clin Oncol 36(6):584–588
Gamez ME, Lal D, Halyard MY et al (2017) Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): the Mayo Clinic experience. Head Neck 39:1819–1824
Amit M, Abdelmeguid AS, Watcherporn T et al (2019) Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J Clin Oncol 37:504–512
London N, Mohyeldin A, Daoud G et al (2020) Sinonasal undifferentiated carcinoma: an institutional trend towards induction chemotherapy followed by definitive chemoradiation. J Neurol Surg B 81(S 01):S1–S272
Krishnamurthy A, Ravi P, Vijayalakshmi R et al (2013) Small cell neuroendocrine carcinoma of the paranasal sinus. Natl J Maxillofac Surg 4(1):111–113
Raychowdhuri RN (1965) Oat-cell carcinoma and paranasal sinuses. J Laryngol Otol 79:253–255
Perez-Ordonez B, Caruana SM, Huvos AG et al (1998) Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol 29:826–832
Fitzek MM, Thornton AF, Varvares M et al (2002) Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer 94(10):2623–2634
Rosenthal DI, Barker JL Jr, El-Naggar AK et al (2004) Sinonasal malignancies with neuroendocrine differentiation. Cancer 101:2567–2573
Brennan SM, Gregory DL, Stillie A et al (2010) Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer 116(4):888–895
Babin E, Rouleau V, Vedrine PO et al (2006) Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinues. J Laryngol Otol 120(4):289–297
Khan M, Nizami S, Mirrahimov AE et al (2014) Primary small cell neuroendocrine carcinoma of the paransal sinuses. Case Rep Med 2014:874719
Hosokawa S, Okamura J, Takizawa Y et al (2013) Long-term survival of a patient with primary small cell neuroendocrine carcinoma of the maxillary sinus: a case report. J Oral Maxillofac Surg 71(8):e248–252
Salhab M, Migdady Y, Donahue M et al (2018) Immunohistochemical expression and prognostic value of PD-L1 in extrapulmonary small cell carcinoma: a single institution experience. J Immunother Cancer 6(1):42
Jethanamest D, Morris LG, Sikora AG et al (2007) Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg 133(3):276–280
Ferlito A, Rinaldo A, Rhys-Evans PH (2003) Contemporary clinical commentary: esthesioneuroblastoma: an update on management of the neck. Laryngoscope 113:1935–1938
Berger L, Luc G, Richard D (1924) The olfactory esthesioneuroepithelioma. Bull Assoc Franc Etude Cancer 13:410–421
Kadish S, Goodman M, Wang CC (1976) Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 37(03):1571–1576
Thompson LDR (2009) Olfactory neuroblastoma. Head and Neck Pathol 3:252–259
Van Gompell JJ, Giannini C, Olsen KD et al (2012) Long-term outcome of esthesioneuroblastoma: hyams grade predicst patient survival. J Neurol Surg B 73(05):331–336
Diaz EM Jr, Johnigan RH III, Pero C et al (2005) Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck 27(02):138–149
Chao KS, Kaplan C, Simpson JR et al (2001) Esthesioneuroblastoma: the impact of treatment modality. Head Neck 23(09):749–757
Resto VA, Eisele DW, Forastiere A et al (2000) Esthesioneuroblastoma: the Johns Hopkins experiene. Head Neck 22(06):550–558
Dulguerov P, Calcaterra T (1992) Esthesioneuroblastoma: the UCLA experience 1970–1990. Laryngoscope 102(08):843–849
Wolfe AR, Blakaj DM, London N et al (2019) Clinical outcomes and multidisciplinary patterns of failure of olfactory neuroblastoma: the Ohio State experience. J Neuro Surg B. https://doi.org/10.1055/s-0039-1692479
Platek ME, Merzianu M, Mashtare TL et al (2011) Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol 6:41
Noh OK, Lee SW, Yoon SM et al (2011) Radiotherapy for esthesioneuroblastoma: is elective nodal irradiation warranted in the multiomodality treatment approach? Int J Radiat Oncol Biol Phys 79(02):443–449
Monroe AT, Hinerman RW, Amdur RJ et al (2003) Radiation therapy for estehsioneuroblastoma: rationale for elective neck irradiation. Head Neck 25(7):529–534
Beitler JJ, Fass DE, Brenner HA et al (1991) Esthesioneuroblastoma: is there a role for elective neck treatment? Head Neck 13:321–326
Demiroz C, Gutfeld O, Aboziada M et al (2011) Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys 81(4):e255–e261
Banuchi VE, Dooley L, Lee NY et al (2016) Patterns of regional and distant metastasis in esthesioneuroblastoma. Laryngoscope 126(7):1556–1561
Wolfe AR, Klamer B, Pan J et al (2018) The impact of adjuvant radiation therapy and elective nodal irradiation in early stage/low grade versus advanced stage/high grade olfactory neuroblastoma. Int J Radiat Oncol Biol Phys 102(3):E360–361
Bauer DE, Mitchell CM, Strait KM et al (2012) Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18(20):5773–5779
Alekseyenko AA, Walsh EM, Zee BM et al (2017) Ectopic protein interactions within BRD4 chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci USA 114(21):E4184–E4192
Grayson AR, Walsh EM, Cameron MJ et al (2014) MYC, a downstream target of BRDNUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene 33(13):1736–1742
Alekseyenko AA, Walsh EM, Wang X et al (2015) The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev 29(14):1507–1523
French CA, Miyoshi I, Kubonishi I et al (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 63(2):304–307
Vargas SO, French CA, Faul PN et al (2001) Upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer 92:1195–1203
Kees UR, Mulcahy MT, Willoughby ML et al (1991) Intrathoracic carcinoma in an 11-year old girl showing translocation t(15;19). Am J Pediatr Hematol Oncol 13:459–464
Lee AC, Kwong YI, Fu KH et al (1993) Disseminated mediastinal carcinoma with chromosomal translocation (15;19): a distinctive clinicopathologic syndrome. Cancer 72:2273–2276
French CA (2010) NUT midline carcinoma. Cancer Genet Cytogenet 203:16–20
Salati M, Baldessari C, Boneti LR et al (2019) NUT midline carcinoma: current concepts and future perspectives of a novel tumour entity. Crit Rev Oncol Hematol 144:102826
Napolitano M, Venturelli M, Molinaro E et al (2019) NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther 12:3235–3244
Giridhar P, Mallick S, Kashyap L et al (2018) Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol 275(3):815–821
Chau NG, Ma C, Danga K et al (2019) An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 4(2):pkz094
Filippakopoulos P, Qi J, Picaud S et al (2010) Selective inhibition of BET bromodomains. Nature 468:1067–1073
Stathis A, Zucca E, Bekradda M et al (2016) Clinical response of carcinomas harboring the BRD4-NUT oncoprotein fo the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov 6:492–500
Piha-Paul SA, Hann CL, French CA et al (2020) Phase 1 study of Molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr 4(2):pkz093
Schwartz BE, Hofer MD, Lemieux ME et al (2011) Differentaition of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 71:2686–2696
Combs SE, Adeberg S, Dittmar JO et al (2013) Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother Oncol 106:186–191
Starke RM, Williams BJ, Hiles C et al (2012) Gamma knife surgery for skull base meningiomas. J Neurosurg 116:588–597
Han J, Girvigian MR, Chen JC et al (2014) A comparative study of stereotactic radiosurgery, hypofractionated, and fractionated stereotactic radiotherapy in the treatment of skull base meningioma. Am J Clin Oncol 37:255–260
El Shafie RA, Czech M, Kessel KA et al (2018) Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol 13:54
Murray FR, Snider JW, Bolsi A et al (2017) Long-term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 99:1190–1198
Rogers CL, Zhang P, Vogelbaum MA et al (2018) Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg 129(1):35–47
Rogers CL, Won M, Vogelbaum MA et al (2020) High-risk meningioma: initial outcomes from NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys 106(4):790–799
Kaley T, Barani I, Chamberlain M et al (2014) Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol 16:829–840
Goldbrunner R, Minniti G, Preusser M et al (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–391
Sahm F, Schrimpf D, Stichel D et al (2017) DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18:682–694
Nigim F, Wakimoto H, Kasper EM et al (2018) Emerging medical treatments for meningioma in the molecular era. Biomedicines 6(3):86
Sklar CA, Antal Z, Chemaitilly W et al (2018) Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol 103(8):2761–2784
Pai HH, Thornton A, Katznelson L et al (2001) Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram anaylisis. Int J Radiat Oncol Biol Phys 49(4):1079–1092
Kyriakakis N, Lynch J, Orme SM et al (2016) Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumors. Clin Endocrinol (Oxf) 84(3):372–379
Children’s Oncology Group (COG) (2018) Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. https://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed Oct 2013
Chemaitilly W, Cohen LE (2017) Diagnosis of endocrine disease: endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol 176(4):R183–R203
Vatner RE, Niemierko A, Misra M et al (2018) Endocrine deficiency as a function of radiation dose to the hypothalamus and pituitary in pediatric and young adult patients with brain tumors. J Clin Oncol 36(28):2854–2862
Garrahy A, Sherlock M, Thompson CJ (2017) Management of endocrine disease: neuroendocrine surveillance and management of neurosurgical patients. Eur J Endocrinol 176(5):R217–R233
VanKoevering KK, Sabetsaverstani K, Sullivan SE et al (2020) Pituitary dysfunction after radiation for anterior skull base malignancies: incidence and screening. J Neurol Surg B 81:75–81
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JDP reports support from Varian Medical Systems, speaking fees from Depuy Synthes and consulting fees from Huron Consulting Group, outside of submitted work. DMT reports clinical trial support from Novocure and publishing fees from Spinger Nature, Inc for projects unrelated to the submitted work. All other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palmer, J.D., Gamez, M.E., Ranta, K. et al. Radiation therapy strategies for skull-base malignancies. J Neurooncol 150, 445–462 (2020). https://doi.org/10.1007/s11060-020-03569-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03569-7