Abstract
Objective
To investigate outcomes after surgery for rare brain tumors using the Swedish Brain Tumor Registry (SBTR).
Methods
This is a nationwide study of patient in the SBTR, validated in the Surveillance, Epidemiology, and End Results (SEER) registries. We included all adults diagnosed 2009–2015 with a rare brain tumor entity (n = 216), defined as ependymoma (EP, n = 64), subependymoma (SUBEP, n = 21), ganglioglioma (GGL, n = 54), pilocytic astrocytoma (PA, n = 56) and primitive neuroectodermal tumor (PNET, n = 21). We analyzed symptomatology, tumor characteristics and outcomes.
Results
Mean age was 38.3 ± 17.2 years in GGL, 36.2 ± 16.9 in PA, 37.0 ± 19.1 in PNET, 51.7 ± 16.3 in EP and 49.8 ± 14.3 in SUBEP. The most common symptom was focal deficit (39.6–71.4%), and this symptom was most common in GGL patients with 64.2% of GGL presenting with seizures. Most patients had no or little restriction in activity before surgery (Performance Status 0–1), although up to 15.0% of PNET patients had a performance status of 4. Gross total resection was achieved in most (> 50%) tumor categories. Incidence of new deficits was 11.1–34.4%. In terms of postoperative complications, 0–4.8% had a hematoma of any kind, 1.9–15.6% an infection, 0–7.8% a venous thromboembolism and 3.7–10.9% experienced a complication requiring reoperation. There were 3 deaths within 30-days of surgery, and a 1-year mortality of 0–14.3%.
Conclusion
We have provided benchmarks for the current symptomatology, tumor characteristics and outcomes after surgery for rare brain tumors as collected by the SBTR and validated our results in an independent registry. These results may aid in clinical decision making and advising patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain tumor surgery is considered a high-risk endeavor [1, 2], especially to consider in the management of rare brain tumors when the personal and even the institutional experience remains limited. Among the ‘rare brain tumors’ are entities such as ganglioglioma (GGL, < 1% of adult intracranial tumors), pilocytic astrocytoma (PA, < 1.5% of adult intracranial tumors), medulloblastoma/primitive neuroectodermal tumors (PNET, < 1.0% of adult tumors), ependymoma (EP, < 1.9% of adult tumors) and subependymoma (SUBEP, < 0.7% of adult intracranial tumors) [3]. Given the rarity of these entities in the adult population, previous reports have mainly focused on the treatment, with systematic data on surgical outcome given little attention [4]. In rare brain tumors, estimates of prognosis and the risks of surgery are likely inaccurate, and certainly less informed than estimates in common tumors. Some of these lesions also have a benign natural course of disease, making it especially important to carefully consider the risk and benefits of surgery given the longevity of this patient population [1]. Even in cases when surgery is unavoidable, the potential of short-term risk is relevant since patients and relatives can be better informed of expected clinical course and potential complications. Well informed patients are likely to cope better when deviations from the optimal post-operative course is encountered.
A limitation for the generalizability of previously few publications is that some are based on non-consecutive and non-population-based material (i.e. patients only treated in selected centers) and are mainly mixed pediatric/adult series [5,6,7,8]. To fill this knowledge gap, we conducted a Swedish nationwide register-based study in the modern era to capture short-term surgical risk profile. We then validate our results in the United States Surveillance, Epidemiology, and End Results (SEER) database.
Materials and methods
The Swedish brain tumor registry
The SBTR is a regionally based registry of adult (18 years or older) patients with diagnosed brain tumors that started in 1999. All regions report to SBTR; however, the level of coverage has varied between the different regions over time. For further details of the registry, see Asklund et al. [9]. The variables registered in the SBTR following surgery are described in detail in Table 1.
Definition of cohort
Using the SBTR, we aimed to include all patients with rare brain tumor entities treated in Sweden from 2009 through 2015 to provide actuality of the current neurosurgical practice. However, in our study we have only used data from regions where the total registration for all tumor entities was 80% or more for any given year to provide population-based data. Registration rate was defined as percentage of diagnoses in the SBTR that corresponds to diagnoses reported to the compulsory National Cancer Registry.
Surveillance, Epidemiology, and End Results (SEER) database
The SEER registry (https://seer.cancer.gov/about/) collects data on cancer incidence and patient survival rates in the United States. We used patient data from SEER database representing age at diagnosis, sex, tumor characteristics (size, laterality, location, World Health Organization (WHO) grade), extent of resection and overall survival for adult patients with medulloblastoma (including PNET), EP, SUBEP, GGL, PA. International Classification of Diseases for Oncology (ICD-O-3) morphology codes were used to define cases: medulloblastoma (including PNET) (9470–9478); EP (9391, 9394); SUBEP (9383); GGL (9505); PA (9421).
Statistics
All analyses of SBTR data were done with SPSS, version 24.0 (Chicago, IL, USA). Statistical significance level was set to p < 0.05. All tests were two-sided. Central tendencies were presented as means ± SD, or median and interquartile range if skewed. Categorical data were analyzed with Pearson´s chi-square test. For survival we presented Kaplan–Meier curves. Data from the SEER database was analyzed using RStudio Version 0.99.467 (RStudio, Boston, Massachusetts, USA).
Results
A total of 226 patients included in the SBTR were identified. Very small subgroups were excluded for further analyses, consequently gangliocytomas (5 patients) and DNET (5 patients) are not part of further analyses that consisted of 216 patients. Among these, there were 54 (25.0%) patients with GGL, 56 (25.9%) patients with PA, 21 (9.7%) patients with the WHO 2007 diagnosis of PNET, 64 (29.6%) patients with EP, and 21 (9.7%) patients with SUBEP.
Ganglioglioma
Mean age was 38.3 ± 17.2 years and there was a slight male predominance (55.6%). The primary locations were frontal (27.8%) and temporal (35.2%). Seizures were present at initial presentation in 64.2% of patients. Only 3.7% of patients were asymptomatic upon presentation, but the majority had no restrictions in their daily activity (WHO performance status 0, 64.2%). Gross total resection was achieved in 63.0% of patients. New deficits occurred in 11.1% after surgery. Two patients (3.7%) had a reoperation due to complication. There were no cases of postoperative hematoma, venous thromboembolism or deaths within 30 days of surgery. For further details see Tables 2 and 3.
Pilocytic astrocytoma
Mean age was 36.2 ± 16.9 years and 26 (46.4%) were female. The primary location was in the cerebellum (37.5%) and focal deficit was a common presenting symptom (50.9%) together with ICP related signs and symptoms (58.5%). Nonetheless, most patients had no restriction on the activities of daily life (WHO performance status 0, 51.8%). Gross total resection was achieved in 55.4% of patients and new deficits occurred in 19.6% after surgery. Three patients (5.4%) had a reoperation due to complication. There was one death (1.8%) within 30 days.
Primitive neuroectodermal tumor
Mean age was 37.0 ± 19.1 years and seven (33.3%) were female. The primary location was in the cerebellum (71.4%) with focal deficits present in most patients (71.4%). Most patients had some restriction on the activities of daily life (WHO performance status I and II, 30.0% each). Gross total resection was achieved in 57.1% of patients and new deficits arose in 33.3% after surgery. There was one (4.8%) reoperation due to complication. There were no deaths within 30 days.
Ependymoma
Mean age was 51.7 ± 16.3 years and 32 (50.0%) were female. The primary location was in the brain stem/4th ventricle (56.3%) with focal deficits in 42.6% of patients (71.4%). Approximately two thirds of patients had no or limited restriction on the activities of daily life (WHO performance status 0 and I, 36.1% and 32.8%). Gross total resection was achieved in 60.9% of patients and new deficits occurred in 34.4% after surgery. Seven patients (10.9%) underwent a reoperation due to complication. There were two deaths (3.1%) within 30 days.
Subependymoma
Mean age was 49.8 ± 14.3 years and 4 (19.0%) were female. The primary location was in the brain stem/4th ventricle (52.4%) with focal deficits in 40.0% of the patients. Most patients had no or limited restriction on the activities of daily life (WHO performance status 0 and I, 40.0% and 50.0%). Gross total resection was achieved in 90.5% of patients and new deficits occurred in 19.0% after surgery. There was one patient (4.8%) that underwent a reoperation due to complication. There were no deaths within 30 days of operation.
Validation in the SEER database
Five thousand, seven hundred, and sixty-nine patients with rare brain tumors were identified in SEER (Table 4). The mean age at diagnosis was 36.4 (GGL), 35.2 (PA), 34.5 (PNET), 46.1 (EP), and 53.3 (SUBEP). While comparable gender distribution was noted for EP, GGL and PA, there was a predominantly male population in Medulloblastoma/PNET and SUBEP. Tumor size at the time of diagnosis was < 4 cm for GGL, PA, EP and SUBEP, while it was 4–6 cm for PNET. Tumors were predominantly unilateral at the time of diagnosis. Almost all (97.4%) medulloblastoma/PNETs and 70.7% of EP were WHO Grade IV and II at the time of diagnosis, respectively, while the majority of SUBEP, GGL and PAs were Grade 1. EP/SUBEP were mainly located in the brain stem and ventricular region; PNET/PA in the cerebellum and GGL in the temporal region. Majority of the cases in each tumor entity underwent gross total resection.
Overall survival SBTR and SEER
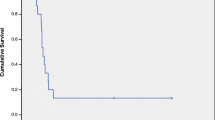
Both SBTR and SEER databases demonstrate SUBEP to have the best overall survival, while Medulloblastoma/PNETs have the worst survival (Fig. 1a and b).
Discussion
In this nationwide registry-based study spanning from 2009–2015, we describe the baseline, tumor and outcome characteristics as well as the 30-day complication rate after intracranial surgery for rare brain tumors in the adult population. To the best of our knowledge, we are the first to systematically report on short-term surgical outcome in terms of morbidity and mortality in adults with rare brain tumors. We provide separate data per entity, and there are variations in short-term outcome among the different tumor entities, commented in detail in subsections below.
Ganglioglioma
The adult literature is scarce, but some data from mixed adult/pediatric cohorts can be used for comparison, although surgical series mainly focus on seizure outcome [5,6,7,8]. Epilepsy is present in 28–85% of patients [4, 5, 10, 11], with the temporal lobe location in up to 62% [5]. Among the adults in our study 64.2% had seizures and 35% of GGL were located in the temporal lobe. Like the literature, we report GTR in > 60% [4, 5, 10], and GTR has been demonstrated to result in better seizure control/freedom than STR or biopsy only [4]. Short term surgical morbidity and mortality is seldom addressed. There are only two recent reports available, demonstrating 1–17% complication/morbidity frequency [12, 13] and another reporting of < 3% mortality [5]. The wide span between the reported complication/morbidity could be caused by the difference in the definition of a complication. In our cohort, new deficits after surgery were reported in 11.1% of cases, with 3.7% in need of re-operation due to complication(s). Finally, whereas adjuvant oncological treatment was planned in 20.8% of our cohort, there is little evidence of adjuvant treatment in other reports, while some even report sporadic or no adjuvant treatment being offered [5, 11], and while the reason for this can only be speculated upon, it could be related to the fact that 11.1% of gangliogliomas in our cohort were anaplastic, while the WHO degree was not disclosed in the other reports, with these possibly comprising only benign (WHO grade 1) entities.
Pilocytic astrocytoma
Treatment outcomes in adult PA has been reported on previously [14,15,16,17,18], albeit mainly with focus on the overall treatment outcome and risk of recurrence rather than surgical morbidity and mortality. Overall, as in our cohort, previous reports describe the mean age of adult pilocytic astrocytoma diagnosis to be in the late 20 s—early 30 s, with localization primarily in the cerebellum and/or brainstem [14,15,16,17,18], with signs and symptoms of raised ICP due to the development of hydrocephalus present in up to 90% in certain reports [16]. Gross total resection has been achieved in approximately 50% of cases in the most modern series [14], comparable to our findings. However, the reported 7% permanent postoperative deficits are markedly lower than in our cohort (19.2%), however there is a difference in reporting where Kamila et al.only reported on permanent deficits [14]. Of note, earlier series by Ye et al. [15] reported up to 20% morbidity, 35% complication frequency and a 10% mortality, which are all higher than numbers reported in our cohort and a possible indication of improvement in surgical care over time. Finally, varying degree of adjuvant treatment has been reported, from 6–22% [14, 15, 17] with our frequency being 9.3%, which is more in line with overall recommendations from recent literature only to use radiotherapy as salvage [19], further substantiated by the demonstration of prolonged PFS but equal OS between adult patients with pilocytic astrocytoma that underwent GTR with- or without adjuvant radiotherapy [20].
Primitive neuroectodermal tumor
PNET stands for primitive neuroectodermal tumor, among which medulloblastoma is the prototype (as defined by the 2007 WHO criteria used in the current SBTR analyses), and as such constitutes most patients reported on [21,22,23]. Reports on outcome in adult medulloblastoma are rare, and in the few that exist, limited or no attention has been given to demographic and surgical characteristics, while focusing on the overall treatment outcome [22, 24,25,26,27,28,29], making comparison difficult.
Those that report on PNET in adults, report a peak incidence in the late 20 s [29], but again, this is highly dependent of whether the patients were considered adults as > 15 y/o or > 18 y/o. In our cohort, the mean age at diagnosis was the late 30 s, consistent with the patients in our cohort included > 18 y/o. The literature describes an up to 20% incidence of metastatic/multifocal disease [27] upon diagnosis, with signs of increased ICP present in the majority of patients [22, 24, 27], similarly to our results with multifocal disease in 23.8% cases and 81% having signs of increased ICP. This correlates well to the severely affected (in comparison to the other rare entities) performance status with 30% of the patients in WHO class I and II, respectively, but even 15% in WHO class IV. In 57.1% GTR was achieved, a frequency that is both lower [21] and higher [27] in literature. Comparing surgical morbidity with the scarce literature is difficult due to non-uniform reporting. We report that 33% of patients experience deficit within 30 days, others have found morbidity in 19% of cases [25]. Further, being a malignant disease with a high symptom burden, there is an indication for timely intervention (all cases operated within < 1 month after radiological diagnosis) as is the need for adjuvant therapy. In the literature almost all reported cases receive adjuvant oncological therapy [21, 22, 28, 29], as was the case in our cohort.
Ependymoma
There are several publications on outcome in adult patients with intracranial ependymomas. The demographic characteristics match these of our cohort; with the patients diagnosed mainly in their late 40 s—early 50 s, presenting with signs/symptoms of raised intracranial pressure, with most tumors located infratentorial and of WHO grade 2 histology [30,31,32,33,34,35]. GTR is reported in 58–74% of cases [34, 35], comparable to our result. Concerning surgical safety profile, as is the case with the other rare entities, the previous reports focus mainly on overall treatment outcome. However, patients with ependymoma in our material experience a high burden of neurological morbidity, but results are comparable to others (27–59%) even though the higher frequency is reported from a mixed intracranial/spinal series [30, 35]. The rather high frequency illustrates that this entity is often located in a sensitive area (56.3% brainstem/4th ventricle location in our cohort) and is approached aggressively in terms of desired GTR. Further, although not previously reported by others, we even report a re-operation frequency of 10.9% and a postoperative infection rate of 15.6%, which is high but comparable to the 11% reported by Acquage et al. [30]. Finally, our perioperative mortality is 3.1%, comparable to 4.7–7.9% reported by others [32, 34].
Subependymoma
SUBEP is a benign (WHO grade 1) tumor, mostly located in the ventricles of the brain, as is the case in our cohort (81% located primarily in one of the four ventricles), with middle aged male preponderance (81% in our cohort) [36,37,38,39,40,41]. There are several recent publications addressing the surgical outcome of SUBEP, albeit mostly small case series, reporting on patients often presenting with headache (> 60%) due to the predominantly ventricular location of these tumors, with risk of secondary hydrocephalus [40, 41]. GTR is usually reported to achieved in > 70% of surgeries [40]. Surgical morbidity of 18–33% [39,40,41] with new focal deficits addressed only by Bi Z et al. as present in 14% of cases [39]. Further, even though mortality is seldom seen, Bi Z et al. reports a 2.3% perioperative mortality [39]. In general, these numbers are in line with the reported frequencies in our cohort, although the frequency of new deficits after surgery is somewhat higher (19%) – which could be associated with > 90% GTR as a sign of more aggressive tumor removal.
Comparison of SEER and SBTR results
While the total patients included from the SBTR is only 216 as compared to 5,769 from the SEER database, the SBTR data provide novel and useful information concerning short-term surgical outcome data in a modern series—data which are not available in the SEER database. Comparing the datasets available in both SEER and SBTR we obtain a gross idea of the clinical profiles of the rare brain tumor patients, and to a large extent the SEER database validates the SBTR. This validation demonstrate that patient and tumor characteristics seem generalizable to other settings. The strength of the SBTR is the national coverage, but also that more clinical and outcome characteristics are available compared to SEER, while the SEER database has a much larger data set and longer follow-up time. We believe that in addition to the baseline characteristics of the studied population, the operative and outcome results can be used as a benchmark for further studies and in improving patient care.
Strengths and limitations
Limitations of this study include those typical of register-based studies with limited details and without possibility to complete missing data since data is provided without identification from the registry holder. Further, there is a lack of detail for variables and long-term follow-up, for instance if the neurological deficit is transient or permanent, as well as considerable possibilities for interpretation of certain variables such as postoperative hematoma.
Strengths include population-based inclusion of a rather large number of patients with rare brain tumors from a recent time period where data is reported in a continuous, prospective and standardized fashion. SEER database has also been analyzed along with the SBTR and found to a large degree to corroborate the SBTR dataset, which increase the external validity our results.
Conclusion
In this nation-wide registry based-study, we benchmark the current symptomatology, tumor characteristics and outcome after intracranial surgery for rare brain tumors—ganglioglioma, pilocytic astrocytoma, primitive neuroectodermal tumor/medulloblastoma, ependymoma and subependymoma—in adult patients in Sweden. This is the largest study focusing on surgical effectiveness and safety when treating rare brain tumors in adult patients, with data from the SBTR to a large extent comparable to those reported in the SEER, increasing the external validity of our results. We believe this information to be of value for both caregivers and patients as to what to expect being operated on due to a rare brain tumor entity.
References
Bartek J Jr, Sjavik K, Forander P et al (2015) Predictors of severe complications in intracranial meningioma surgery: a population-based multicenter study. World Neurosurg 83(5):673–678
Wong JM, Panchmatia JR, Ziewacz JE et al (2012) Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus 33(5):E16
Gittleman HR, Ostrom QT, Rouse CD et al (2015) Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 121(1):102–112
Luyken C, Blumcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J (2004) Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer 101(1):146–155
Im SH, Chung CK, Cho BK et al (2002) Intracranial ganglioglioma: preoperative characteristics and oncologic outcome after surgery. J Neurooncol 59(2):173–183
Isimbaldi G, Sironi M, Tonnarelli GP, Roncoroni M, Declich P, Galli C (1996) Ganglioglioma: a clinical and pathological study of 12 cases. Clin Neuropathol 15(4):192–199
Haddad SF, Moore SA, Menezes AH, VanGilder JC (1992) Ganglioglioma: 13 years of experience. Neurosurgery 31(2):171–178
Song JY, Kim JH, Cho YH, Kim CJ, Lee EJ (2014) Treatment and outcomes for gangliogliomas: a single-center review of 16 patients. Brain Tumor Res Treat 2(2):49–55
Asklund T, Malmstrom A, Bergqvist M, Bjor O, Henriksson R (2015) Brain tumors in Sweden: data from a population-based registry 1999–2012. Acta Oncol 54(3):377–384
Yust-Katz S, Anderson MD, Liu D et al (2014) Clinical and prognostic features of adult patients with gangliogliomas. Neuro Oncol 16(3):409–413
Compton JJ, Laack NN, Eckel LJ, Schomas DA, Giannini C, Meyer FB (2012) Long-term outcomes for low-grade intracranial ganglioglioma: 30-year experience from the Mayo Clinic. J Neurosurg 117(5):825–830
Hu WH, Ge M, Zhang K, Meng FG, Zhang JG (2012) Seizure outcome with surgical management of epileptogenic ganglioglioma: a study of 55 patients. Acta Neurochir 154(5):855–861
Tandon V, Bansal S, Chandra PS et al (2016) Ganglioglioma: single-institutional experience of 24 cases with review of literature. Asian J Neurosurg 11(4):407–411
Bond KM, Hughes JD, Porter AL, Orina J, Fang S, Parney IF (2018) Adult pilocytic astrocytoma: an institutional series and systematic literature review for extent of resection and recurrence. World Neurosurg 110:276–283
Ye JM, Ye MJ, Kranz S, Lo P (2014) A 10 year retrospective study of surgical outcomes of adult intracranial pilocytic astrocytoma. J Clin Neurosci 21(12):2160–2164
Bell D, Chitnavis BP, Al-Sarraj S, Connor S, Sharr MM, Gullan RW (2004) Pilocytic astrocytoma of the adult–clinical features, radiological features and management. Br J Neurosurg 18(6):613–616
Johnson DR, Brown PD, Galanis E, Hammack JE (2012) Pilocytic astrocytoma survival in adults: analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol 108(1):187–193
Stuer C, Vilz B, Majores M, Becker A, Schramm J, Simon M (2007) Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer 110(12):2799–2808
Brown PD, Anderson SK, Carrero XW et al (2015) Adult patients with supratentorial pilocytic astrocytoma: long-term follow-up of prospective multicenter clinical trial NCCTG-867251 (Alliance). Neurooncol Pract 2(4):199–204
Ishkanian A, Laperriere NJ, Xu W et al (2011) Upfront observation versus radiation for adult pilocytic astrocytoma. Cancer 117(17):4070–4079
Lai R (2008) Survival of patients with adult medulloblastoma: a population-based study. Cancer 112(7):1568–1574
Smee RI, Williams JR (2008) Medulloblastomas-primitive neuroectodermal tumours in the adult population. J Med Imaging Radiat Oncol 52(1):72–76
Menon G, Krishnakumar K, Nair S (2008) Adult medulloblastoma: clinical profile and treatment results of 18 patients. J Clin Neurosci 15(2):122–126
Kunschner LJ, Kuttesch J, Hess K, Yung WK (2001) Survival and recurrence factors in adult medulloblastoma: the M.D. Anderson Cancer Center experience from 1978 to 1998. Neuro-Oncology 3(3):167–173
Hadi I, Roengvoraphoj O, Niyazi M et al (2018) Medulloblastoma in adults : a retrospective single institution analysis. Strahlenther Onkol 194(3):225–234
Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M (2007) Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 110(9):2035–2041
Lai SF, Wang CW, Chen YH et al (2012) Medulloblastoma in adults. Treatment outcome, relapse patterns, and prognostic factors. Strahlenther Onkol 188(10):878–886
Riffaud L, Saikali S, Leray E et al (2009) Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg 111(3):478–487
Abacioglu U, Uzel O, Sengoz M, Turkan S, Ober A (2002) Medulloblastoma in adults: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys 54(3):855–860
Acquaye AA, Vera E, Gilbert MR, Armstrong TS (2017) Clinical presentation and outcomes for adult ependymoma patients. Cancer 123(3):494–501
Armstrong TS, Vera-Bolanos E, Gilbert MR (2011) Clinical course of adult patients with ependymoma: results of the Adult Ependymoma Outcomes Project. Cancer 117(22):5133–5141
Dutzmann S, Schatlo B, Lobrinus A et al (2013) A multi-center retrospective analysis of treatment effects and quality of life in adult patients with cranial ependymomas. J Neurooncol 114(3):319–327
Metellus P, Guyotat J, Chinot O et al (2010) Adult intracranial WHO grade II ependymomas: long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro-Oncology 12(9):976–984
Metellus P, Barrie M, Figarella-Branger D et al (2007) Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain 130(Pt 5):1338–1349
Bostrom A, von Lehe M, Hartmann W et al (2011) Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery. 68(2):302–308 discussion 309
Ragel BT, Osborn AG, Whang K, Townsend JJ, Jensen RL, Couldwell WT (2006) Subependymomas: an analysis of clinical and imaging features. Neurosurgery 58(5):881–890 discussion 881-890
Rushing EJ, Cooper PB, Quezado M et al (2007) Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol 85(3):297–305
Nguyen HS, Doan N, Gelsomino M, Shabani S (2017) Intracranial subependymoma: a SEER analysis 2004–2013. World Neurosurg 101:599–605
Bi Z, Ren X, Zhang J, Jia W (2015) Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg 122(1):49–60
Kandenwein JA, Bostroem A, Feuss M, Pietsch T, Simon M (2011) Surgical management of intracranial subependymomas. Acta Neurochir 153(7):1469–1475
Jain A, Amin AG, Jain P et al (2012) Subependymoma: clinical features and surgical outcomes. Neurol Res 34(7):677–684
Acknowledgements
Open access funding provided by Karolinska Institute. This project was made possible by the continuous work of the Swedish Brain Tumor Registry (SBTR), Roger Henriksson (chairman), Thomas Asklund, Annika Malmström, Lena Damer, Lena Rosenlund, Rickard Sjöberg, Sofia Hylin, Peter Milos, Thomas Blystad, Sara Kinhult, Göran Hesselager, Petra Witt Nyström, Katja Werlenius, Asgeir S. Jakola, Gregor Tomasevic, Magnus Olivecrona, Margret Jensdottir, Michael Bergqvist, Marie Sjögren, Eskil Degsell, Linnea Nilsson, Kerstin Rehn, Kristina Lundqvist, and Lisa Tykosson.
Funding
This study was financed through ALF-medel (ALF-GBG 716671) and the Swedish Research Council (2017–00944).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The ethical committee in Western Sweden approved the research project (Dnr: 363–17) and waived the need for informed consent according to the Swedish Law. The registry holder also approved the study.
Research involved in human or animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartek, J., Dhawan, S., Thurin, E. et al. Short-term outcome following surgery for rare brain tumor entities in adults: a Swedish nation-wide registry-based study and comparison with SEER database. J Neurooncol 148, 281–290 (2020). https://doi.org/10.1007/s11060-020-03490-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03490-z