Abstract
Purpose
Dynamic susceptibility contrast (DSC) MR-perfusion is becoming a standard of care for the monitoring of glioblastoma. Yet, technical standards are lacking and measurements without leakage correction are still common. Also, data on leakage corrected measurements during stable disease is scarce. In this study we hypothesized that basic leakage correction would significantly enhance data quality during stable disease and improve progress detection. We furthermore investigated whether longitudinal data could increase diagnostic performance.
Methods
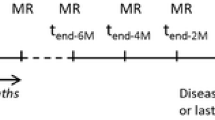
Patients with histologically proven glioblastoma undergoing first-line therapy were prospectively recruited. We conducted DSC perfusion measurements without prebolus administration in 6-week intervals from the end of radiotherapy until progression. Maximum relative cerebral volume values (rCBVmax) with and without leakage correction were calculated using Philips IntelliSpace®.
Results
We recruited 16 patients and conducted 82 MRI scans with a mean follow up of 7.2 month. During stable disease, corrected rCBVmax was significantly more stable than uncorrected rCBVmax. Detection of progression with a rCBVmax cutoff was better for corrected (specificity 86%) than for uncorrected rCBVmax (specificity 41%). Interestingly, the increase of corrected rCBVmax upon progression also had a good diagnostic performance with a combination of both cutoffs delivering the best result (sensitivity/specificity 89%/93%).
Conclusion
Corrected rCBVmax supports the imaging finding of a stable disease and large increases during longitudinal observation support the diagnosis of tumor progression. rCBV values without prebolus or leakage correction are not reliable to monitor glioblastomas. Further studies to investigate the value of longitudinal rCBV dynamics for the differentiation of real tumor progression from pseudoprogression are warranted.




Similar content being viewed by others
References
Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M et al (2019) Lomustine–temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): a randomised, open-label, phase 3 trial. Lancet 393:678–688. https://doi.org/10.1016/S0140-6736(18)31791-4
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Lombardi G, de Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M et al (2019) Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 20:110–119. https://doi.org/10.1016/S1470-2045(18)30675-2
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48:2192–2202. https://doi.org/10.1016/j.ejca.2012.04.011
Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M et al (2015) Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro-oncology 17:1188–1198. https://doi.org/10.1093/neuonc/nov095
Patel P, Baradaran H, Delgado D, Askin G, Christos P, John Tsiouris A, Gupta A (2017) MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro-oncology 19:118–127. https://doi.org/10.1093/neuonc/now148
Boxerman JL, Ellingson BM, Jeyapalan S, Elinzano H, Harris RJ, Rogg JM et al (2017) Longitudinal DSC-MRI for distinguishing tumor recurrence from pseudoprogression in patients with a high-grade glioma. Am J Clin Oncol 40:228–234. https://doi.org/10.1097/COC.0000000000000156
Blasel S, Zagorcic A, Jurcoane A, Bähr O, Wagner M, Harter PN, Hattingen E (2016) Perfusion MRI in the evaluation of suspected glioblastoma recurrence. J Neuroimaging 26:116–123. https://doi.org/10.1111/jon.12247
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Leu K, Boxerman JL, Ellingson BM (2017) Effects of MRI protocol parameters, preload injection dose, fractionation strategies, and leakage correction algorithms on the fidelity of dynamic-susceptibility contrast MRI estimates of relative cerebral blood volume in gliomas. AJNR Am J Neuroradiol 38:478–484. https://doi.org/10.3174/ajnr.A5027
Bell LC, Hu LS, Stokes AM, McGee SC, Baxter LC, Quarles CC (2017) Characterizing the influence of preload dosing on percent signal recovery (PSR) and cerebral blood volume (CBV) measurements in a patient population with high-grade glioma using dynamic susceptibility contrast MRI. Tomography 3:89–95. https://doi.org/10.18383/j.tom.2017.00004
Pullicino R, Radon M, Biswas S, Bhojak M, Das K (2018) A review of the current evidence on gadolinium deposition in the brain. Clin Neuroradiol 28:159–169. https://doi.org/10.1007/s00062-018-0678-0
Leu K, Boxerman JL, Cloughesy TF, Lai A, Nghiemphu PL, Liau LM et al (2016) Improved leakage correction for single-echo dynamic susceptibility contrast perfusion MRI estimates of relative cerebral blood volume in high-grade gliomas by accounting for bidirectional contrast agent exchange. AJNR Am J Neuroradiol 37:1440–1446. https://doi.org/10.3174/ajnr.A4759
Haselhorst R, Kappos L, Bilecen D, Scheffler K, Möri D, Radü EW, Seelig J (2000) Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: application of an extended blood-brain barrier leakage correction. J Magn Reson Imaging 11:495–505. https://doi.org/10.1002/(SICI)1522-2586(200005)11:5%3c495:AID-JMRI5%3e3.0.CO;2-S
Bell LC, Semmineh N, An H, Eldeniz C, Wahl R, Schmainda KM et al (2019) Evaluating multisite rCBV consistency from DSC-MRI imaging protocols and postprocessing software across the NCI quantitative imaging network sites using a digital reference object (DRO). Tomography 5:110–117. https://doi.org/10.18383/j.tom.2018.00041
Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M (2015) ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am J Neuroradiol 36:E41–51. https://doi.org/10.3174/ajnr.A4341
Hu LS, Kelm Z, Korfiatis P, Dueck AC, Elrod C, Ellingson BM et al (2015) Impact of software modeling on the accuracy of perfusion MRI in glioma. AJNR Am J Neuroradiol 36:2242–2249. https://doi.org/10.3174/ajnr.A4451
Hedderich D, Kluge A, Pyka T, Zimmer C, Kirschke JS, Wiestler B, Preibisch C (2019) Consistency of normalized cerebral blood volume values in glioblastoma using different leakage correction algorithms on dynamic susceptibility contrast magnetic resonance imaging data without and with preload. J Neuroradiol 46:44–51. https://doi.org/10.1016/j.neurad.2018.04.006
Jafari-Khouzani K, Emblem KE, Kalpathy-Cramer J, Bjørnerud A, Vangel MG, Gerstner ER et al (2015) Repeatability of cerebral perfusion using dynamic susceptibility contrast MRI in glioblastoma patients. Transl Oncol 8:137–146. https://doi.org/10.1016/j.tranon.2015.03.002
Schmainda KM, Prah MA, Rand SD, Liu Y, Logan B, Muzi M et al (2018) Multisite concordance of DSC-MRI analysis for brain tumors: results of a National Cancer Institute Quantitative Imaging Network Collaborative Project. AJNR Am J Neuroradiol 39:1008–1016. https://doi.org/10.3174/ajnr.A5675
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Müller A, Jurcoane A, Kebir S, Ditter P, Schrader F, Herrlinger U et al (2017) Quantitative T1-mapping detects cloudy-enhancing tumor compartments predicting outcome of patients with glioblastoma. Cancer Med 6:89–99. https://doi.org/10.1002/cam4.966
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 7:e51862. https://doi.org/10.1371/journal.pone.0051862
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Thust SC, Heiland S, Falini A, Jäger HR, Waldman AD, Sundgren PC et al (2018) Glioma imaging in Europe: a survey of 220 centres and recommendations for best clinical practice. Eur Radiol 28:3306–3317. https://doi.org/10.1007/s00330-018-5314-5
Pedersen M, Klarhöfer M, Christensen S, Ouallet JC, Ostergaard L, Dousset V et al (2004) Quantitative cerebral perfusion using the PRESTO acquisition scheme. J Magn Reson Imaging 20(6):930–940. https://doi.org/10.1002/jmri.20206
Acknowledgements
We thank our medical technical radiology assistants who participated in performing the MRI scans for this study.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Ethics Committee of the Medical Faculty of the University of Bonn, Reference: Lfd.Nr. 349/14) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Steidl, E., Müller, M., Müller, A. et al. Longitudinal, leakage corrected and uncorrected rCBV during the first-line treatment of glioblastoma: a prospective study. J Neurooncol 144, 409–417 (2019). https://doi.org/10.1007/s11060-019-03244-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03244-6