Abstract
Purpose
To demonstrate that lesions of the visual pathways due to suprasellar tumors are accompanied by alterations of the visual cortex and to see if these alterations are reversible after treatment of tumors by gamma knife radiosurgery.
Materials and methods
In 36 patients with peri-optic tumors and defects of their visual fields and in an age-matched control group, magnetic resonance imaging was performed before and after treatment. T1 weighted images were evaluated by voxel-based morphometry and correlated to the degree of visual field defects.
Results
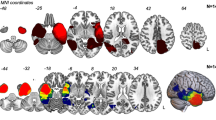
In patients, grey matter density and cortical thickness were reduced in all parts of the occipital cortex, reaching significance (p < 0.05) in the left superior and middle occipital gyri, with correlation to visual field defects. Follow-up scans showed further reduction in all occipital areas.
Conclusion
As in other peripheral lesions of the optic system, damage of the optic pathways affects the visual cortex. A prospective follow-up study is needed to determine if these alterations are reversible after successful tumor treatment.

Similar content being viewed by others
Abbreviations
- CC:
-
Correlation coefficient
- GKRS:
-
Gamma knife radiosurgery
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- AD:
-
Axial diffusivity
- RD:
-
Radial diffusivity
- HF-SRS:
-
Hypo-fractionated stereotactic radiosurgery
- AVP:
-
Anterior visual pathway
- SFED:
-
Single fraction equivalent dose
- TIV:
-
Total intracranial volume
References
Boucard CC, Hernowo AT, Maguire RP, Jansonius NM, Roerdink JB, Hooymans JM et al (2009) Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain 132:1898–1906. https://doi.org/10.1093/brain/awp119
Frezzotti P, Giorgio A, Motolese I, De Leucio A, Iester M, Motolese E et al (2014) Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS ONE 9:e105931. https://doi.org/10.1371/journal.pone.0105931
Prins D, Plank T, Baseler HA, Gouws AD, Beer A, Morland AB et al (2016) Surface-based analyses of anatomical properties of the visual cortex in macular degeneration. PLoS ONE 11:e0146684. https://doi.org/10.1371/journal.pone.0146684
Brown HD, Woodall RL, Kitching RE, Baseler HA, Morland AB (2016) Using magnetic resonance imaging to assess visual deficits: a review. Ophthalmic Physiol Opt 36:240–265. https://doi.org/10.1111/opo.12293
Burge WK, Griffis JC, Nenert R, Elkhetali A, DeCarlo DK, ver Hoef LW et al (2016) Cortical thickness in human V1 associated with central vision loss. Sci Rep 6:23268. https://doi.org/10.1038/srep23268
Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH (2006) Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90:674–678
Barcella V, Rocca MA, Bianchi-Marzoli S, Milesi J, Melzi L, Falini A et al (2010) Evidence for retrochiasmatic tissue loss in Leber’s hereditary optic neuropathy. Hum Brain Mapp 31:1900–1906. https://doi.org/10.1002/hbm.20985
Mendola JD, Conner IP, Roy A, Chan ST, Schwartz TL, Odom JV et al (2005) Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp 25:222–236
Lemos J, Pereira D, Castelo-Branco M (2016) Visual cortex plasticity following peripheral damage to the visual system: fMRI evidence. Curr Neurol Neurosci Rep 16:89. https://doi.org/10.1007/s11910-016-0691-0
Lou AR, Madsen KH, Julian HO, Toft PB, Kjaer TW, Paulson OB et al (2013) Postoperative increase in grey matter volume in visual cortex after unilateral cataract surgery. ActaOphthalmol 91:58–65. https://doi.org/10.1111/j.1755-3768.2011.02304.x
Shao Y, Keliris GA, Papanikolaou A, Fischer MD, Zobor D, Jägle H et al (2013) Visual cortex organisation in a macaque monkey with macular degeneration. Eur J Neurosci 38:3456–3464. https://doi.org/10.1111/ejn.12349
Vernimmen FJ, Slabbert JP (2010) Assessment of the alpha/beta ratios for arteriovenous malformations, meningiomas, acoustic neuromas, and the optic chiasma. Int J Radiat Biol 86:486–498
Dahnke R, Yotter R, Gaser C (2012) Cortical thickness and central surface estimation. Neuroimage 65:336–348
Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL et al (2007) Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage 39:1064–1080. https://doi.org/10.1016/j.neuroimage.2007.09.031
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. https://doi.org/10.1016/j.neuroimage.01.021
Fischer E, Bülthoff HH, Logothetis NK, Bartels A (2012) Visual motion responses in the posterior cingulate sulcus: a comparison to V5/MT and MST. Cereb Cortex 22:865–76. https://doi.org/10.1093/cercor/bhr154
Williams AL, Lackey J, Wizov SS, Chia TM, Gatla S, Moster ML et al (2013) Evidence for widespread structural brain changes in glaucoma: a preliminary voxel-based MRI study. Invest Ophthalmol Vis Sci 54:5880–5887
Hernowo AT, Prins D, Baseler HA, Plank T, Gouws AD, Hooymans JM et al (2014) Morphometric analyses of the visual pathways in macular degeneration. Cortex 56:99–110. https://doi.org/10.1016/j.cortex.2013.01.003
Keenan TDL, Goldacre R, Goldacre MJ (2014) Associations between age-related macular degeneration, Alzheimer disease, and dementia. JAMA Ophthalmol 132:63–68
Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T et al (1997) MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol 18:1291–1295
Cheung SH, Legge GE (2005) Functional and cortical adaptations to central vision loss. Vis Neurosci 22:187–201
Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R et al (2017) Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci 37:5065–5073. https://doi.org/10.1523/JNEUROSCI.3550-16.2017
Guinan EM, Lowy C, Stanhope N, Lewis PD, Kopelman MD (1998) Cognitive effects of pituitary tumours and their treatments: two case studies and an investigation of 90 patients. J NeurolNeurosurg Psychiatry 65:870–876
Gaser C, Schlaug G (2003) Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci 999:514–517
Anurova I, Renier LA, De Volder AG, Carlson S, Rauschecker JP (2015) Relationship between cortical thickness and functional activation in the early blind. Cereb Cortex 25:2035–2048. https://doi.org/10.1093/cercor/bhu009
Yuste R, Bonhoeffer T (2004) Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci 5:24–34
Voss P, Pike BG, Zatorre RJ (2014) Evidence for both compensatory plastic and disuse atrophy-related neuroanatomical changes in the blind. Brain 137:1224–1240. https://doi.org/10.1093/brain/awu030
Gilbert CD, Li W (2012) Adult visual cortical plasticity. Neuron 75:250–264. https://doi.org/10.1016/j.neuron.2012.06.030
Author information
Authors and Affiliations
Contributions
Conception and design: Peter Stoeter, Herwin Speckter. Data collection: Jose Bido, Remberto Escoto, Cesar Gonzalez, Giancarlo Hernandez, Jairo Oviedo, Diones Rivera, Luis Suazo, Santiago Valenzuela, Peter Stoeter, Herwin Speckter. Data analysis and interpretation: Jose Bido, Remberto Escoto, Bernd Foerster, Cesar Gonzalez, Giancarlo Hernandez, Jairo Oviedo, Diones Rivera, Luis Suazo, Santiago Valenzuela, Herwin Speckter, Peter Stoeter. Manuscript writing: Peter Stoeter, Herwin Speckter, Remberto Escoto. Final approval of manuscript: Jose Bido, Remberto Escoto, Bernd Foerster, Giancarlo Hernandez, Jairo Oviedo, Diones Rivera, Luis Suazo, Santiago Valenzuela, Cesar Gonzalez, Peter Stoeter, Herwin Speckter.
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study has been approved by the ethic committee of CEDIMAT (CEI-290).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Speckter, H., Bido, J., Hernandez, G. et al. Adaptation of visual cortex to damage of visual pathways in suprasellar tumors before and after gamma knife radiosurgery. J Neurooncol 142, 275–282 (2019). https://doi.org/10.1007/s11060-019-03092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03092-4