Abstract
Subject
To date there is no established tumor marker for the clinical follow-up of glioblastoma, WHO grade IV, (GBM) which constitutes the most frequent and malignant primary brain tumor. However, since there is promising data that the serum glial fibrillary acidic protein (sGFAP) may serve as a biomarker for glial brain tumors, this prospective study aimed at evaluating the diagnostic relevance of perioperative changes in sGFAP levels for the assessment of residual glial tumor tissue in patients undergoing surgery of intracerebral tumors.
Methods
Serum GFAP was measured using an electrochemiluminometric immunoassay (ElecsysR GFAP prototype test, Roche Diagnostics, Penzberg/Germany) in 32 prospectively recruited patients between September 2009 and August 2010. Twenty-five were diagnosed with glioma and seven with brain metastases (BM). We assessed sGFAP levels prior to and at different time points during the early postoperative phase until patient discharge.
Results
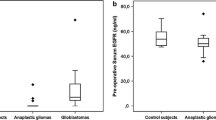
There were only significant differences in the pre-operative sGFAP levels of patients with gliomas compared to BM (0.18 vs. 0.08 µg/l; p = 0.0198, Welch’s t-Test). Even though there was an increase of sGFAP after surgery, there were no significant differences between glioma and BM patients at any other time point. Peak sGFAP levels where reached on postoperative day 1 followed by a slight decrease, but not reaching pre-operative levels until postop day 7. There was no significant correlation between postoperative glioma tumor volume and sGFAP levels in univariate analyses.
Conclusion
According to our data sGFAP does not appear to be suitable to detect residual glioma tissue in the acute postoperative phase.



Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ et al (2012) A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro-Oncology. https://doi.org/10.1093/neuonc/nos087
Khalifa J, Tensaouti F, Chaltiel L, Lotterie J-A, Catalaa I, Sunyach MP et al (2016) Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol. https://doi.org/10.1007/s00330-016-4234-5
Buchmann N, Kläsner B, Gempt J, Bauer JS, Pyka T, Delbridge C et al (2016) (18) F-fluoroethyl-L-thyrosine (FET) PET to delineate tumor residuals after glioblastoma resection: a comparison to standard postoperative MRI. World Neurosurg. https://doi.org/10.1016/j.wneu.2016.02.032
Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4615
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841. https://doi.org/10.1148/radiol.13131669
Siddall JK, Shetty SD, Cooper EH (1986) Measurements of serum gamma-seminoprotein and prostate specific antigen evaluated for monitoring carcinoma of the prostate. Clin Chem 32:2040–2043
Seamonds B, Yang N, Anderson K, Whitaker B, Shaw LM, Bollinger JR (1986) Evaluation of prostate-specific antigen and prostatic acid phosphatase as prostate cancer markers. Urology 28:472–479
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E (1987) Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909–916. https://doi.org/10.1056/NEJM198710083171501
Eng LF (1986) Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol 8:203–214
Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO et al (2001) Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7:839–845. https://doi.org/10.1158/1078-0432.ccr-06-0181
Kiviniemi A, Gardberg M, Frantzén J, Parkkola R, Vuorinen V, Pesola M et al (2015) Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: correlation to tumor volume, molecular markers, and progression-free survival. J Neuro-Oncol 124:237–245. https://doi.org/10.1007/s11060-015-1829-7
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25:1439–1451
Yung WK, Luna M, Borit A (1985) Vimentin and glial fibrillary acidic protein in human brain tumors. J Neuro-Oncol 3:35–38
Jung CS, Foerch C, Schänzer A, Heck A, Plate KH, Seifert V et al (2007) Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 130:3336–3341. https://doi.org/10.1093/brain/awm263
Brommeland T, Rosengren L, Fridlund S, Hennig R, Isaksen V (2007) Serum levels of glial fibrillary acidic protein correlate to tumour volume of high-grade gliomas. Acta Neurol Scand 116:380–384. https://doi.org/10.1111/j.1600-0404.2007.00889.x
Lyubimova NV, Toms MG, Popova EE, Bondarenko YV, Krat VB, Kushlinskii NE (2001) Neurospecific proteins in the serum of patients with brain tumors. Bull Exp Biol Med 150:732–734
Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A et al (2012) Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 58:237–245. https://doi.org/10.1373/clinchem.2011.172676
Tichy J, Spechtmeyer S, Mittelbronn M, Hattingen E, Rieger J, Senft C et al (2015) Prospective evaluation of serum glial fibrillary acidic protein (GFAP) as a diagnostic marker for glioblastoma. J Neuro-Oncol 126:361–369. https://doi.org/10.1007/s11060-015-1978-8
Husain H, Savage W, Grossman SA, Ye X, Burger PC, Everett A et al (2012) Pre- and post-operative plasma glial fibrillary acidic protein levels in patients with newly diagnosed gliomas. J Neuro-Oncol 109:123–127. https://doi.org/10.1007/s11060-012-0874-8
Raheja A, Sinha S, Samson N, Bhoi S, Subramanian A, Sharma P et al (2016) Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled Phase II clinical trial. J Neurosurg. https://doi.org/10.3171/2015.6.JNS15674
Condorelli DF, Dell’Albani P, Kaczmarek L, Messina L, Spampinato G, Avola R et al (1990) Glial fibrillary acidic protein messenger RNA and glutamine synthetase activity after nervous system injury. J Neurosci Res 26:251–257. https://doi.org/10.1002/jnr.490260216
Mucke L, Oldstone MB, Morris JC, Nerenberg MI (1991) Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. N Biologist 3:465–474
Arai N (1992) The role of swollen astrocytes in human brain lesions after edema–an immunohistochemical study using formalin-fixed paraffin-embedded sections. Neurosci Lett 138:56–58
Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA et al (2013) Invasion patterns in brain metastases of solid cancers. Neuro-Oncol 15:1664–1672. https://doi.org/10.1093/neuonc/not112
Neves S, Mazal PR, Wanschitz J, Rudnay AC, Drlicek M, Czech T et al (xxxx) Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin Neuropathol 20:38–42
Shaffrey ME, Mut M, Asher AL, Burri SH, Chahlavi A, Chang SM et al (2004) Brain metastases. Curr Prob Surg 41:665–741. https://doi.org/10.1067/j.cpsurg.2004.06.001
Baumert BG, Rutten I, Dehing-Oberije C, Twijnstra A, Dirx MJM, Debougnoux-Huppertz RMTL. et al (2006) A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiation Oncol Biol Phys 66:187–194. https://doi.org/10.1016/j.ijrobp.2006.03.050
Kamp MA, Dibué M, Niemann L, Reichelt DC, Felsberg J, Steiger H-J et al (2012) Proof of principle: supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir 154:1981–1986. https://doi.org/10.1007/s00701-012-1463-5
Kamp MA, Dibué M, Santacroce A, Zella SM, Niemann L, Steiger H-J et al (2013) The tumour is not enough or is it? Problems and new concepts in the surgery of cerebral metastases. Ecancermedicalscience 7:306. https://doi.org/10.3332/ecancer.2013.306
Kamp MA, Rapp M, Slotty PJ, Turowski B, Sadat H, Smuga M et al (2015) Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir 157:905-10-1. https://doi.org/10.1007/s00701-015-2405-9
Acknowledgements
We thank Marina Heibel for excellent technical support. MM would like to thank the Luxembourg National Research Fond (FNR) for the support (FNR PEARL P16/BM/11192868 Grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baumgarten, P., Quick-Weller, J., Gessler, F. et al. Pre- and early postoperative GFAP serum levels in glioma and brain metastases. J Neurooncol 139, 541–546 (2018). https://doi.org/10.1007/s11060-018-2898-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2898-1