Abstract
Purpose
Magmas (mitochondria-associated protein involved in granulocyte–macrophage colony-stimulating factor signal transduction) is a nuclear gene that encodes the mitochondrial import inner membrane translocase subunit Tim16. Magmas is highly conserved, ubiquitously expressed in mammalian cells, and is essential for cell viability. Magmas expression levels are increased in prostate cancers and pituitary adenomas. Moreover, silencing Magmas by RNAi sensitizes pituitary adenoma cells to pro-apoptotic stimuli and induces a G0/G1 accumulation. The aim of this study was to examine whether inhibition of Magmas by small molecule inhibitors could be beneficial for the treatment of malignant gliomas.
Methods
We evaluated the expression of Magmas in patient-derived glioblastoma tissue samples and xenograft models. We studied the feasibility of a small molecule Magmas inhibitor (BT#9) as a therapeutic agent in stable human glioma cell lines and high-grade patient derived glioma stem-like cells.
Results
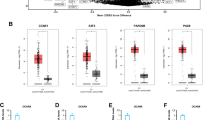
Magmas was overexpressed in tissue sections from glioma patients and xenografts. In vivo studies revealed that BT#9 could cross the blood–brain barrier in the animal model. Magmas inhibition by BT#9 in glioma cell lines significantly decreased cell proliferation, induced apoptosis along with vacuole formation, and blocked migration and invasion. In addition, BT#9 treatment decreased the respiratory function of glioma cells, supporting the role that Magmas serves as a reactive oxygen species regulator.
Conclusions
This is the first study on the role of Magmas in glioma. Our findings suggest that Magmas plays a key role in glioma cell survival and targeting Magmas by small molecule inhibitors may be a therapeutic strategy in gliomas.





Similar content being viewed by others
References
Jubinsky PT, Messer A, Bender J, Morris RE, Ciraolo GM, Witte DP et al (2001) Identification and characterization of Magmas, a novel mitochondria-associated protein involved in granulocyte-macrophage colony-stimulating factor signal transduction. Exp Hematol 29:1392–1402
Jubinsky PT, Short MK, Mutema G, Witte DP (2003) Developmental expression of Magmas in murine tissues and its co-expression with the GM-CSF receptor. J Histochem Cytochem 51:585–596
Peng J, Huang CH, Short MK, Jubinsky PT (2005) Magmas gene structure and evolution. In Silico Biol 5:251–263
Jubinsky PT, Short MK, Mutema G, Morris RE, Ciraolo GM, Li M (2005) Magmas expression in neoplastic human prostate. J Mol Histol 36:69–75
Tagliati F, Gentilin E, Buratto M, Mole D, degli Uberti EC, Zatelli MC (2010) Magmas, a gene newly identified as overexpressed in human and mouse ACTH-secreting pituitary adenomas, protects pituitary cells from apoptotic stimuli. Endocrinology 151:4635–4642
Tagliati F, Gagliano T, Gentilin E, Minoia M, Mole D, Delgi Uberti EC et al (2013) Magmas overexpression inhibits staurosporine induced apoptosis in rat pituitary adenoma cell lines. PLoS ONE 8:e75194
Srivastava S, Sinha D, Saha PP, Marthala H, D’Silva P (2014) Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis 5:e1394
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12
Kim SS, Harford JB, Pirollo KF, Chang EH (2015) Effective treatment of glioblastoma requires crossing the blood-brain barrier and targeting tumors including cancer stem cells: the promise of nanomedicine. Biochem Biophys Res Commun 468:485–489
Pistollato F, Chen HL, Rood BR, Zhang HZ, D’Avella D, Denaro L et al (2009) Hypoxia and HIF1α repress the differentiative effects of BMPs in high-grade glioma. Stem Cells 27:7–17
Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L et al (2013) Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA 110:8644–8649
Jubinsky PT, Short MK, Ghanem M, Das BC (2011) Design, synthesis, and biological activity of novel Magmas inhibitors. Bioorg Med Chem Lett 21:3479–3482
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, Kostrov SV (2016) Cytoplasmic vacuolization in cell death and survival. Oncotarget 7:55863–55889
Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S et al (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 9:424–429
Frazier AE, Dudek J, Guiard B, Voos W, Li Y, Lind M et al (2004) Pam16 has an essential role in the mitochondrial protein import motor. Nat Struct Mol Biol 11:226–233
Kozany C, Mokranjac D, Sichting M, Neupert W, Hell K (2004) The J domain-related cochaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nat Struct Mol Biol 11:234–241
Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M et al (2002) Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21:6082–6090
Bayley JP, Devilee P (2010) Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr Opin Genet Dev 20:324–329
Gillies RJ, Gatenby RA (2007) Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr 39:251–257
Brat DJ, Castellano-Sanchez A, Kaur B, Van Meir EG (2002) Genetic and biologic progression in astrocytomas and their relation to angiogenic dysregulation. Adv Anat Pathol 9:24–36
Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH et al (2004) Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 64:920–927
Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT et al (2004) Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 10:8177–8184
Acknowledgements
This study was supported by donations from grateful patients to Dr. Bota laboratory and the UCI Cancer Center Award Number P30CA062203 from the National Cancer Institute (NCI/NIH). Dr. Das is thankful to Dr. Michael Baltezor (Director, Biotechnology Innovation and Optimization Center, Kansas City, KS) for the PK studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interests.
Rights and permissions
About this article
Cite this article
Di, K., Lomeli, N., Bota, D.A. et al. Magmas inhibition as a potential treatment strategy in malignant glioma. J Neurooncol 141, 267–276 (2019). https://doi.org/10.1007/s11060-018-03040-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03040-8