Abstract
Forest development following agricultural abandonment concerns extensive areas including the Massif Central region of France where this study was undertaken. This land-use and land-cover change is expected to have effects on biodiversity and ecosystem services, including an increase of carbon sequestration—a major concern in the face of climate change. Nevertheless, uncertainties about carbon stock changes during successions are remaining, especially as to the total stock and the contribution of the different carbon pools. Our work contributes to this field by studying carbon stocks in multiple plots of different successional stages. We measured and estimated carbon stocks in aboveground and belowground vegetation, deadwood, litter and superficial soil, and surveyed plant communities and plot conditions (slope, aspect, soil characteristics). The average total carbon stock increased along the succession from 70.60 at stage 0 to 314.19 tC ha−1 at stage 5. However, the total carbon stocks at the young forest stage (abandoned for 74 years maximum) and the older forest stage (forested for at least 74 years) were not significantly different, and probably reflected strong local heterogeneity in the older forest stage. An increase of the carbon stock was found in all pools, except the soil pool that did not vary significantly between the successional stages. The aboveground carbon stock was found strongly related to the woody species cover, especially the macrophanerophyte cover. This case study supports the view that the succession dynamics of former agricultural plots participates in carbon sequestration, sometimes with great local variations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural abandonment is a major land use and land cover (LULC) change in Europe along with urbanization and agricultural intensification (Gerard et al. 2010; van Vliet et al. 2015). Following the cessation of agricultural activity, vegetation succession leads to old fields going through various successional stages (Lepart and Escarré 1983), except under harsh biotic or abiotic constraints. Under temperate climates, succession occurs via a transition from open ecosystems dominated by herbaceous plant species to shrubs, and finally forested ecosystems (Cramer and Hobbs 2007). Agricultural abandonment concerns extensive areas worldwide (Chazdon et al. 2020), with an estimated 150 million hectares abandoned between 1700 and 1992 (Ramankutty and Foley 1999) and still around 60 million hectares between 2003 and 2019 (Potapov et al. 2022). The trend is expected to continue: Perpiña Castillo et al. (2021) predicted that some 5.6 million hectares are likely to be abandoned by 2030 (3.6% of 2015 agricultural zones). Abandonment leads to an increase of forest cover (Rudel et al. 2005; Meyfroidt and Lambin 2011) including in the Massif Central region of France where this study was led (CEREMAC 2000).
Agricultural abandonment clearly impacts biodiversity through changes in vegetation composition and structure, and changes in other related taxa like insects (Dantas de Miranda et al. 2019) and birds (Fonderflick et al. 2010). In their review of the ecological impacts of agricultural changes in Europe, Stoate et al. (2009) report that vegetation development after abandonment altogether tends to have a positive effect on biodiversity associated to shrub and forested ecosystems but negative effects on farmland biodiversity. The changes occurring along secondary successions, i.e. shifts from herb-dominated communities to shrubs and trees (Cramer and Hobbs 2007), come together with changes in ecosystem functioning (Cramer and Hobbs 2007; Romero-Díaz et al. 2017), and effects on ecosystem services (ES) (Lasanta et al. 2015; Leal Filho et al. 2017; Ustaoglu and Collier 2018). Ecosystem services are the benefits delivered by ecosystems to humans (Millennium Ecosystem Assessment 2005) stemming from their biological properties and functioning (Villamagna et al. 2013). Abandonment may also lead to ecosystem disservices (EDS), i.e. the harmful and negative inputs from ecosystems (Lyytimaki & Sipila 2009; von Döhren & Haase 2015; Blanco et al. 2020). Various effects of abandonment on ES and EDS have been reported in the literature, such as increased forestry production, increased fire risks, and positive impacts on “water, soil and air protection, and carbon sequestration” (Stoate et al. 2009).
The potential impact of abandonment on carbon sequestration represents a crucial issue in the face of climate change, which jeopardizes the living conditions of human beings, e.g. with increased catastrophic climate events (IPCC 2022). Climate change is also an essential issue for biodiversity as it is expected to reduce the habitat ranges of plant and animal species, generates stresses, and increases mortality (Raj et al. 2018; IPCC 2022; Vacek et al. 2023). Defined as the “regulation of chemical composition of atmosphere and oceans” by ecosystems (code 2.2.6.1 of the Common International Classification of Ecosystem Services (V5.1) (Haines-Young and Potschin 2018)) carbon sequestration mitigates the effects of climate change by decreasing greenhouse gases in the atmosphere. The expected increase of the carbon stock with vegetation development along succession on former agricultural lands can efficiently participate to carbon sequestration (Ziegler et al. 2012; Bastin et al. 2019). Such an increase of the carbon stock is all the more needed in France, where carbon sequestration objectives are lagging behind (Haut Conseil pour le Climat 2022).
Ecological evaluation and quantification of ES is necessary to inform management decisions (de Groot et al. 2002) and to map and accurately model ES (Vigerstol and Aukema 2011; Bartholomée et al. 2018; Meraj et al. 2022). From a quantitative point of view, estimations of carbon stock changes after abandonment are still uncertain. Carbon stock changes through spontaneous succession have been studied, e.g. litter decomposition along successional gradients (Mayer 2008) and the carbon stocks of aboveground vegetation in Spanish forests since the 1950s (Vilà-Cabrera et al. 2017). Yet, to our knowledge, only a few studies have investigated a variety of stages including plots under agricultural use and old forests, while also looking at multiple carbon pools (aboveground, necromass, belowground and superficial soil) (Hooker and Compton 2003; Risch et al. 2008; Aryal et al. 2014; Badalamenti et al. 2019; Facioni et al. 2019; Jones et al. 2019; Finzi et al. 2020; Thibault et al. 2022), and none has focused on west-European temperate forests. Such an approach is important because the carbon stock changes during spontaneous vegetation growth are less controlled and straightforward than those that occur in forest plantations: diverse vegetation pathways and local conditions induce variations in carbon accumulation (Benjamin et al. 2005; Foote and Grogan 2010; Kalt et al. 2019; Smith et al. 2020).
Following abandonment, the total amount of carbon stored in an ecosystem is expected to increase as a consequence of biomass accumulation in vegetation, litter and soil (Hooker and Compton 2003). Succession stages differ from each other by their carbon pools, in absolute stock (de Jesus Silva et al. 2016; Badalamenti et al. 2019; Facioni et al. 2019; Gogoi et al. 2020) as well as in the relative contribution of the different pools (aboveground vegetation, roots, and soil) (Bartholomée et al. 2018). These contrasts are expected because the belowground-to-aboveground biomass ratio is lower in trees than in grasses and shrubs (IPCC 2006a, b; Freschet et al. 2017), and aboveground living biomass tends to dominate the carbon stock in late successional stages (Badalamenti et al. 2019). The last stages of old-grown forests are expected to have the highest stock, even if the carbon stored of old-grown forests vary between biomes (Pan et al. 2011; Keith et al. 2014; Badalamenti et al. 2019).
In temperate regions, agricultural abandonment is followed by a change in species composition from light-demanding plant species to shade-tolerant ones (Harmer et al. 2001). The shift in dominating life forms from herbaceous (therophyte, geophyte, hemicryptophyte) to woody perennial (chamaephyte, phanerophyte) may be key in explaining changes in the carbon stock. However, the community composition varies within and among stages (Morel et al. 2020; Ciurzycki et al. 2021) and can bring additional effects on carbon sequestration patterns, e.g. through dominant tree species in communities (Vallet et al. 2009; Vesterdal et al. 2013) and species canopy structure (Hardiman et al. 2013). Carbon stock may also be influenced by plant diversity: more diversified communities tend to enhance niche complementarity and may increase plant aboveground biomass (Loreau and Hector 2001; Li et al. 2017). Along with successional stages and species composition, local factors can affect the carbon stock. Clay-rich soils tend to retain more nutrients (Manrique et al. 1991; Lu et al. 2002) and nutrient-richer soils support higher vegetation growth (Moran et al. 2000); slope is also a determining factor: steeper slopes tend to increase water drainage and soil nutrient loss (Robinson et al. 2015).
To contribute to these challenges, we evaluated carbon sequestration during vegetation successions following abandonment by carrying out field measurements and distinguishing the patterns within various successive vegetation stages. Our aim was also to study the relationships between plant biodiversity, ecosystem functioning and ES (Walker et al. 2010; Prach and Walker 2011). Accordingly, we investigated the relationship between (i) the carbon stock and (ii) successional stages and local environmental conditions. We studied post-agricultural plots belonging to different successional stages and situated in temperate low mountains of central France. We conducted field measurements of the carbon stock to address the three following questions: (i) how much carbon is stored in old fields and forests in total?; (ii) how does the carbon stock change among the different pools during succession?; and (iii) which local biotic and abiotic factors influence the carbon stock in old fields and forests? We tested the following hypotheses: (a) the total carbon store increases with succession, and forest stages store amounts of carbon similar to those measured in other temperate forests, i.e. around 346 tC ha−1 (Finzi et al. 2020) to 373 tC ha−1 (Lecointe et al. 2006); (b) all above- and below-ground carbon pools increase along the vegetation succession, but their relative contribution to the carbon stock (%) varies between stages; (c) the carbon store varies depending on multiple factors including the species composition of the plant communities, their Raunkiaer life forms (Raunkiaer 1934), richness and diversity, the soil type and its nitrogen content, and the slope aspect and inclination.
Materials and methods
Study area
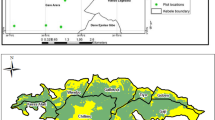
The study was conducted in a 166-km2 area (45° 40′ N, 3° 06′ E) situated in the Massif Central region of France (Fig. 1A), south of Clermont-Ferrand conurbation, at the junction between mountainous systems of volcanic origin in the west (Chaîne des Puys and Massif des Monts Dore) and a sedimentary basin in the east (Limagne plain, the Allier River alluvial plain). The area comprised granitic and volcanic plateaus and low mountains in the west, a plain in the east made of volcanic and limestone-clay colluvial deposits, and a series of west-to-east valleys in-between (Fig. 1B). Mean annual temperature varies between 8.7 and 11.6 °C, and mean annual precipitation between 578.9 and 796.1 mm (Roux 2017).
Characteristics of the study area. A Localization of the study area in France. B Elevation contrasts, rivers, and urban and artificial areas of the study area at present. C Shrub and forest covers in 2019. Black, forests already present in 1946; dark green and light green, forests and shrubs having developed between 1946 and 2019, respectively, indicating agricultural abandonment followed by vegetation succession and forest development
Agricultural abandonment has been taking place in the area for at least two centuries. We used 13 historical orthophotographs of the study area (1946, 1962, 1974, 1978, 1981, 1984, 1986, 1989, 1999–2001, 2004–2009, 2016 and 2019) retrieved from the National Institute of Geographic and Forest Information (IGN) covering a roughly 70-year-long period to determine the historical record of the area and of its agriculture. The analysis of the photographs indicated that 21% of agricultural lands in 1946 had been abandoned and colonized by ligneous vegetation by 2019; shrub, wood, and mature forest covers went from 19 to 34% of the study area during the same period (Weissgerber et al. 2022) (Fig. 1C). In 1946, the main agricultural activities were arboriculture, viticulture, cattle and sheep farming, and subsistence agriculture, versus croplands (cereals, oil seeds) and pastureland in 2019.
Determination of successional stages in the study area and plot selection
Plot selection for carbon stock measurements was based on successional stages (Table 1). Stages were first identified on aerial photographs, and then plots from five successional stages—plus a first stage (thereafter called ‘0’) corresponding to grazed plots still under agricultural use—were selected after field observations and description (Fig. 2). Three shrub-rich stages (1, 2, 3) were abandoned but unwooded, with an increasing shrub cover at the expense of herbaceous vegetation from stage 1 to stage 3. Stage 4 corresponded to young forests in plots unforested in 1946 but forested in 2020, when field work was conducted. On the basis of the orthophotographs, the corresponding plots had been forested (visible crowns) at least since 1999–2001. The oldest stage (stage 5) corresponded to plots that showed visible crowns and were already forested on the 1946 orthophotographs. These forests were at least 74 years old in 2020. These plots had been under agricultural use at the beginning of the nineteenth century according to the ‘Napoleon’ land register established between 1808 and 1833 in the region, so we may assume that their abandonment and reforestation happened between 1833 and 1946.
Pictures showing examples of plots typical of the five studied successional stages. Number in the bottom left corner, successional stage (see text). Plots of stages 1 and 2 include two vegetation types. H, herbaceous vegetation type; W, woody vegetation type (see Table 1)
Agricultural activity greatly changed in the area during the last two centuries. Data on the agricultural history of each abandoned plot were limited and mostly derived from the historical aerial photographs, and we had approximately one per 10-year period since 1946. Moreover, succession rates vary depending on multiple causes including soil quality and distance to the seed source (Lepart and Escarré 1983; Acácio et al. 2007; Rühl and Schnittler 2011; Ciurzycki et al. 2021), so that abandonment periods can only be determined with low precision. As a result, we adopted semi-quantitative classes based on successional stages (0, 1, 2, 3, 4, 5). We selected stage 1, 2, 3, 4 plots formerly managed as grasslands (not as croplands or vineyards or orchards), but the land use of some plots may have changed several times in the decades before their abandonment.
Five plots were selected per successional stage. All plots were situated on valley sides, at the center of the study area, within a 2.3-km radius (Appendix A). Soils were primarily brown soils (Callot et al. 1966; Hétier 1973) (approximately equivalent to Cambisols in the WRB) that overlaid a bedrock composed of a variety of volcanic rocks and limestone-clay colluvial deposits. Twenty subplots (19 in two cases, see Table 1) were delimited in each plot. Two types of subplots were distinguished to capture the heterogeneity of stages 1 and 2: one was dominated by herbaceous vegetation (H), and the other was dominated by woody vegetation (W) (Table 1, Fig. 2). This distinction between 1H and 1W, and 2H and 2W was maintained throughout the study. The subplot size varied to fit the spatial structure of the stages (16 m2 for stages 0–1-2–3, 36 m2 for stage 4, 100 m2 for stage 5).
Vegetation surveys
The surveys were conducted on the subplots. As stated, their size varied according to the vegetation type (Gillet 1998; Andrade et al. 2019). We pursued observational objectives to characterize plant communities rather than the completeness of their species composition (Andrade et al. 2019). The vascular plant species composition of each subplot was recorded in spring and early summer 2020 (Tison and Foucault 2014). The species cover was determined visually as a percentage of the subplot total area. All vegetation strata (herbs, shrubs, trees) were surveyed, and the total species cover potentially exceeded 100% on the plots harboring several vegetation strata. Vegetation surveys were not conducted on stage 0 plots because regular grazing disturbed plant development.
Measurements of the aboveground carbon stock
The aboveground carbon stock in living biomass was measured and estimated according to the vegetation form, i.e. grasses, shrubs, or trees (Table 3).
The aboveground biomass of herbaceous plants was measured by determining (i) the relationship between grass height (cm) and grass mass per surface unit (g cm−2), and (ii) the average grass height of each subplot. The relationship between grass height and grass mass per surface unit was established by sampling herbaceous vegetation (400 cm2 of known height) in each subplot and weighing it after drying at 60 °C for at least 24 h; all values were plotted, and the resulting equation was B = 0.24 · H + 4.98, where B is the herbaceous aboveground biomass (g cm−2) and H is herbaceous vegetation height (cm). The average grass height of each subplot was determined by measuring grass height every 15 cm on the diagonal of the subplot, i.e. 37 points in 16-m2 subplots.
Shrub biomass was estimated from the volume of vegetation using the equation from Montero et al. (2013):
where W is the biomass (tons of material per hectare), Hm is the average shrub height (dm), and FCCm is the proportion of the subplot covered by shrubs.
Tree biomass was estimated from the diameter at breast height (DBH) of trees following species-specific equations of the following form:
where B is the aboveground biomass (kg) and D the DBH (cm). When species-specific equations were not available, the general equation following Zianis and Mencuccini (2004), and Bartholomée et al. (2018) was used, i.e.:
The detailed equations are available in appendix B.
Three types of aboveground dead biomass were considered: standing dead trees, fallen dead trees, and litter lying on the soil. The biomass of standing dead trees was calculated with the general equation following Zianis and Mencuccini (2004), and Bartholomée et al. (2018), i.e.:
where B is the aboveground biomass (kg) and D the DBH (cm).
The biomass of fallen dead trees was estimated from tree diameter and height. Tree density decreases after death. Consequently, a density of 0.26 g cm−3 was chosen to determine biomass from volume, following Bartholomée et al. (2018). Litter biomass was determined by sampling litter on 400 cm2 in each subplot and weighing after drying it at 60 °C for 24 h.
We used ratios to determine carbon contents. For all living vegetation types, the carbon content equated to 0.47 · biomass (IPCC 2006b). We also applied a constant of 0.44 · biomass to determine litter carbon, based on Chojnacky et al. (2009) and Bartholomée et al. (2018). However, the litter carbon content is variable, so this caveat should be borne in mind when interpreting the results.
Estimation of the belowground carbon stock
Root biomass was estimated from aboveground biomass using root-to-shoot ratios (Table 2) (IPCC 2006a, b; Mokany et al. 2006; Bartholomée et al. 2018).
Measurements of the soil carbon stock
Two types of soil samples were collected at two soil depths (0–10 cm and 10–20 cm) in each subplot (Table 3) (four samples per subplot). The first sample type was obtained using a standard stainless-steel 5 cm × 5 cm cylinder and was used to determine soil bulk density (g cm−3), following Peco et al. (2017) and Bartholomée et al (2018). Each soil sample was weighed, all roots and stones were carefully removed and weighed, and then all samples were sifted through a 2-mm sieve, dried at 105 °C for 24 h, and weighed. Multiple mass measurements allowed us to ensure that no soil had been lost in the process. For the second sample type, soil from each subplot was placed in a sealable plastic bag. The samples were pooled per plot after careful mixing, and sent to the Agronomic Laboratory of Normandie to measure organic carbon (g kg−1) (NF ISO 14235), nitrogen (g kg−1) (NF ISO 11261) and determine texture (NF X31-107).
The soil organic carbon (SOC) content (g cm−3) was determined as the organic carbon content multiplied by the bulk density.
Data analysis
A principal component analysis (PCA) on the Hellinger-transformed species-cover matrix (Legendre and Legendre 2012) was performed to compare the vegetation species composition among the different successional stages and vegetation types. Only the plant species with a cumulative cover > 5% in the dataset were taken into account.
The differences in total carbon stock and carbon stored in each pool between the successional stages and the vegetation types were tested using one-way analysis of variance (ANOVA; P < 0.05), followed by Student’s post-hoc test when conditions were met. Otherwise, Kruskal–Wallis test were applied, followed by Dunn’s post-hoc test with Benjamini-Hochfeld adjustment (Lee and Lee 2018; Jafari and Ansari-Pour 2019).
To investigate which environmental variables explained carbon stock variations, we used generalized linear mixed models (GLMMs, with a gaussian error distribution) with ‘plot’ as a random effect (lme4 package; Bates et al. 2019). Multiple explanatory variables were considered (Table 4). Variables were not scaled to ensure a better interpretability of estimates. The initial model was as follows: aboveground carbon tC ha−1 ~ species PCA dim1 + species PCA dim2 + Macrophanerophyte cover + Mesophanerophyte cover + Microphanerophyte cover + Nanophanerophyte cover + Geophyte cover + Therophyte cover + Hemicryptophyte cover + Chamaephyte cover + richness + Shannon index + soil nitrogen (g kg−1) + cos(aspect) + sin(aspect) + slope inclination + soil clay + soil sand + soil silt + soil depth + (1 | plot).
We produced all candidate models testing all combinations of variables up to a maximum of 4 variables per model to limit the complexity of the models and computation time. Then, we used a multi-model inference approach to calculate model-averaged parameter estimates (with 95% confidence intervals) for the variables included in the selected models (cumulated weight AICc < 0.95, Akaike information criterion corrected for small sample sizes, Burnham and Anderson 2002). The dredging and model averaging procedures were performed using the MuMin package (Bartoń 2016). The effect of an explanatory variable was considered significant when its estimate (the slope of the relation) was different from zero (when its 95% confidence interval excluded zero). The quality of the models was evaluated using the r2 values calculated for the fixed effects for the global model (marginal r2 using the “r.squaredGLMM” function in the MuMin package; Bartoń 2016).
The tests were performed using Excel and Rstudio (R version 4.1.0).
Results
Vegetation composition of the surveyed plots
The vegetation composition varied between successional stages (Fig. 3), even if similarities in species composition were found between the herbaceous plant communities of stages 1 and 2 (1H and 2H), and the woody shrub communities of stage 1 (1W). Indeed, the plant communities of stages 1H, 2H and 1W were all dominated by Bromus erectus (Huds.) and Brachypodium pinnatum (L.). By contrast, stages 2W and 3 were dominated by Cornus sanguinea (L.). Stage 4 was dominated by Fraxinus excelsior (L.) and Robinia pseudoacacia (L.), while stage 5 was dominated by Quercus robur (L.) and Abies alba (Mill.). Some species were present in both last stages, especially Hedera helix (L.) but also Acer campestre (L.) and Corylus avenala (L.). However, the compositions of these two stages globally differed: stage 4 still included a high cover of Prunus avium (L.), Crataegus sp. and Cornus sanguinea (L.), while stage 5 included a higher cover of Fagus sylvatica (L.) and Carpinus betulus (L.). Therefore, the plant species composition at stage 4 – corresponding to agricultural plots abandoned for approximately 65–75 years –, was different from that of older forests.
Total carbon stock: an increase with succession but heterogeneity at the last stage
The total carbon stock increased from stage 0 to stage 5, and was multiplied by 4.5 on average (Fig. 4). The total carbon stocks of the woody vegetation types of stages 1 and 2 (1W and 2W) were not significantly different, but they differed from the stocks of the herbaceous communities (1H and 2H). The average total carbon stocks were 216.80 tC ha−1 in stage 4, and 314.19 tC ha−1 in stage 5, with high variation between subplots (min. 125.71 tC ha−1; max. 535.82 tC ha−1).
Carbon pools: aboveground biomass drives the increase of the total carbon stock, but no variation of the soil stock
The carbon stored in aboveground vegetation varied between 1.10 tC ha−1 minimum at stage 0 (2.68 tC ha−1 on average) and 326.60 tC ha−1 maximum at stage 5 (175.36 tC ha−2 on average) (Table 5). Estimations of the belowground (root) carbon stock ranged from 3.00 at stage 0 to 117.14 tC ha−1 at stage 3. The amount of carbon stored in the belowground vegetation of stage 3 was not significantly different from the stock in the woody vegetation of stages 1 (1W) and 2 (2W) or from the overall amounts of stages 4 and 5. The carbon stored in litter and deadwood increased with the successional stages, and the greatest stock was found at stage 5. No significant difference in superficial SOC emerged between the different stages.
Clear differences appeared between the herbaceous and woody vegetation types within stages 1 and 2: although the soil pool stored the most carbon in both stages, the development of shrubs brought about an important increase of the aboveground and belowground carbon stocks. In the last stages of the succession (stages 4 and 5), aboveground vegetation was the largest carbon pool (Table 5, Fig. 5).
Variations of the aboveground carbon stock: determining factors
The relationship between carbon stocks and biotic and abiotic variables was tested considering the aboveground living biomass pool. The stocks of the other pools were considered to show the same pattern because their carbon stores were correlated (appendix C). Modeling indicated that the aboveground carbon stock was positively related to the macrophanerophyte, mesophanerophyte and chamaephyte covers, and negatively related to the plant species composition as approached by the first axis of the PCA (Table 6 and Fig. 3). The PCA first axis captured changes in the herbaceous species cover (more positive scores) and tree species (more negative scores). This shows that the plant community composition has a major effect on carbon stocks, beyond the effect of the successional stage. In particular, the presence of bigger, late-successional tree species was related to higher carbon stocks.
Discussion
Vegetation succession and total carbon stocks in the plots
We measured the carbon stocks of many plots representing five successional stages, plus one stage still under agricultural use (grazing) in an area concerned by agricultural abandonment in central France.
As expected, carbon stocks increased along the succession. Furthermore, vegetation surveys showed that the species composition varied along the successional stages following succession models (Lepart and Escarré 1983; Cramer and Hobbs 2007). Early stages were characterized by herbaceous species and dominated by poaceae. Then, shrub species colonized the plots, followed by pioneer trees (Fraxinus excelsior, Robinia pseudoacacia). After approximately 65–75 years of development, young forests had not reached the composition of older forests dominated by oak and fir. Holmes et al. (2018) showed similar tendencies for forest herb communities that differed from those of old forest communities 80 years after abandonment.
As hypothesized, the total amounts of carbon stored in abandoned plots increased along with the successional stages. However, the differences between stages were not all significant, and in particular the carbon stocks of forest stages 4 and 5 were not significantly different. This is in line with Yue et al. (2018) who studied forests of various ages in northwestern China, but contradicts Badalamenti et al. (2019) who found a higher carbon stock in old Mediterranean forests compared to young forests. Literature shows that the timespan for forest biomass to recover after abandonment is variable (Aryal et al. 2014). Stages 4 (< 74 years) and 5 (> 74 years) had globally similar total carbon stocks but different vegetation compositions. Our results are not in agreement with Bauters et al. (2019) who studied tropical forest of the central Congo Basin and found a quick recovery of species diversity and functional composition but a lag in carbon storage during secondary succession.
Regarding the amount of carbon stored in the forest stages, we hypothesized that the forests of the study area would store carbon in similar amounts to other forests in Europe and temperate regions. As expected, young forests (stage 4) stored between 122.09 tC ha−1 and 403.93 tC ha−1 (216.80 tC ha−1 on average), and older forests (stage 5) between 125.71 tC ha−1 and 535.82 tC ha−1 (314.19 tC ha−1 on average). When looking at the total carbon stock of forests following spontaneous post-abandonment succession, Finzi et al. (2020) found 345.73 ± 53.82 tC ha−1 in an 80–120 year-old forest (oak, maple, hemlock) in central Massachusetts (USA), while Badalamenti et al. (2019) found nearly 150 tC ha−1 in a young forest (Mediterranean forest-maquis) and about 198 tC ha−1 in an old-growth forest, in the same range as the stocks estimated in the present study. In France, many studies concern planted forests, but our mean values remain close to those stocks. For instance, Lecointe et al. (2006) found 230.5 ± 14.6 tC ha−1 in 15–60 year-old stands of broad-leaved forests, that increased up to 373.0 ± 36.9 tC ha−1 in > 90-year-old stands. In central Italy, Facioni et al. (2019) found a higher stock: nearly 600 tC ha−1 in new woods and nearly 700 tC ha−1 on average in old woods, but it should be noted that those woods were managed coppice, which may explain the higher stock. As for a larger-scale approach, the French General Committee for Sustainable Development (Commissariat général au développement durable 2019) estimated that the French continental forest stored 730 tC ha−1, while Pan et al (2011) found a stock of 155 tC ha−1 in temperate forests based on forest inventory data and long-term ecosystem carbon studies. Such a large variation in estimations suggest heterogeneity in temperate forests carbon stock, while probably also related to methodological differences.
The carbon stocks of successional stages 4 and 5 were quite heterogenous, which may explain why these stages were not significantly different. Some of the mentioned studies do find a high heterogeneity as well (Facioni et al. 2019) while others only consider one site per stage (Badalamenti et al. 2019). This heterogeneity in the forested stages we studied could be attributable to climate contrasts among plots (e.g. rainfall variability) (Facioni et al. 2019; Velázquez et al. 2022) or slope aspect (Sharma et al. 2011). Further work in our study area should be solely focused on forests to examine the factors of carbon stock variation within these stages.
Contribution of the different carbon pools to the total carbon stock
The relative contributions of the carbon pools (aboveground, belowground, soil) to the total carbon stock (%) were expected to vary between the successional stages. They varied accordingly and to a large extent throughout the succession, following plant community changes. From stage 0 to stage 3, the highest proportion of carbon was in the soil, while carbon was predominantly stored in aboveground vegetation at stages 4 and 5 (Table 5, Fig. 5). Dominance of aboveground vegetation in the carbon stock has been reported: vegetation-stored carbon has been found higher than soil-stored carbon only in the last stage of the succession (oak dominated old-growth forest more than 105 years old (Badalamenti et al. 2019) and dry semi-deciduous mature secondary forest (Robinson et al. 2015)); in the Swiss Alps, Risch et al. (2008) measured a higher carbon stock in the belowground pool (soil and roots) than in the aboveground pool (125 ± 16 tC ha−1 vs. 90 ± 6 tC ha−1, respectively) in a young mountain pine forest, but the opposite in an older mixed conifer forest (107 ± 7 tC ha−1 vs. 166 ± 24 tC ha−1, respectively). Badalamenti et al. (2019) also found that aboveground vegetation stored 53.1% of carbon in the older forest stage, in line with our results (55.81%). Moreover, we observed that nearly 10% of the carbon was stored in deadwood in stage 5 while litter carbon stock varies only slightly during succession, but such result should be considered carefully as we did not measure litter carbon content directly.
The greatest accumulation of biomass during the succession was in aboveground living vegetation (min. 1.10 tC ha−1; max. 326.60 tC ha−1), in accordance with results obtained in the Mediterranean area (Badalamenti et al. 2019) and in the east of Brazil (Matos et al. 2020). In our study area, stage 5 was characterized by a significantly higher carbon stock in aboveground vegetation compared to stage 4, and this fits with a difference observed between a 35 year-old secondary forest and an old forest (Aryal et al. 2014).
Although we expected all carbon pools to increase along the vegetation succession, the superficial soil pool did not. The absence of a SOC pattern along the succession contrasts with the majority of other studies, which show either an increasing SOC with succession related to an increased biomass input (Zornoza et al. 2009; Foote and Grogan 2010; Piché and Kelting 2015; Chang et al. 2017; Nunes 2019; Lasanta et al. 2020) or a higher SOC content in undisturbed older forests compared to young forests due to land-use legacy (Yesilonis et al. 2016). However, SOC does not always increase during forest regrowth after abandonment (Alberti et al. 2008; Gosheva et al. 2017); Alberti et al. (2008) attributed variations in SOC across sites to precipitation, vegetation type, previous land uses, and a time-lag for litter changes to impact the soil carbon content, while Gosheva et al. (2017) attributed these variations to climate, soil chemistry and tree species. In tree plantations on agricultural lands, a worldwide meta-analysis showed that SOC changes depend on the previous land use, and that increases are detected when cropland—not grasslands—are afforested (Shi et al. 2013); moreover, an analysis of 619 sites in China showed that the overall mean change in SOC density was not significant, but SOC density increased when the initial SOC density was low, and vice versa (Hong et al. 2020). These trends could explain why we did not observe any significant SOC variation. The absence of a superficial SOC increase with succession may also have been due to a rapid turnover of organic matter that limited its accumulation, as found by Yan et al. (2006). This result on the soil carbon stock calls for an extended analysis of the soil nutrient content (e.g. nitrogen) and plot temperature and moisture because they are important factors of the carbon dynamic (Post and Kwon 2000; Marín-Spiotta and Sharma 2013; Sierra et al. 2015; Palmer et al. 2019).
The absence of significant SOC changes along stages may also be explained by our sampling strategy. We only considered superficial soil (0-20cm) because it tends to contain the greatest proportion of SOC (Batjes 1996; Jobbagy and Jackson 2000). But this may have led us to underestimate the soil carbon stock, as deeper soil horizons also participate to the carbon stock (Rumpel and Kögel-Knabner 2011; Shi et al. 2013; Jackson et al. 2017). Moreover, we did not use the equivalent soil mass procedure for SOC quantification (Wendt and Hauser 2013). This may have influenced our results (Guidi et al. 2014), possibly overestimating SOC (Wendt and Hauser 2013).
Drivers of the aboveground carbon stock
The vegetation composition played a main role in the carbon stock increase along the succession through the positive effect on aboveground carbon stock of woody species. The presence of large trees greatly influenced the aboveground carbon stock, as already reported by Lecointe et al. (2006) in western France, Måren & Sharma (2021) in Nepal and Dimobe et al. (2019) in eastern Burkina Faso, who showed that big trees (DBH ≥ 25 cm) stored 70% of the total aboveground carbon.
Contrary to the expectations of Robinson et al. (2015), we found no relationship between the carbon stock and the slope, possibly due to the absence of very steep slopes in the plots (ranging from 2° to 19°). Our results neither showed relationship between the aboveground carbon stock and soil nutrients (the nitrogen concentration), in accordance with Holl and Zahawi (2014) and contrary to Moran (2000). This may indicate that the soil nutrient content is not a limiting factor for forest regrowth in the study area.
Our results altogether show a limited relationship between local conditions and the aboveground carbon stock in a large range of successional stages, but major effects related to the vegetation composition and the late successional species.
Conclusion
We measured carbon stored in ecosystems after agricultural abandonment. Multiple successional stages were considered, from grazed plots (stage 0) to a forest at least 74 years old (stage 5). Several carbon pools were measured: aboveground living and dead biomass, belowground biomass (roots), and superficial soil.
As hypothesized, the amount of carbon stored in old fields increased with the successional stages: the last forest stage reached 314.19 tC ha−1 on average. Younger and older forest stages stored amounts of carbon similar to those stored by other forests in temperate Europe, but quite remarkably our results show great variability within stages. The relative contribution of each pool to the carbon stock varies between successional stages, and aboveground living biomass drives the carbon stock increase in the last stages. In contrast, the carbon stored in the soil did not increase with the succession. Stored carbon was related to successional stages and large trees, but carbon stock changes throughout the succession was not significantly related to slope or to the soil clay and nitrogen contents.
Further research in our study area should concentrate on detailing the soil dynamic and investigating the drivers of the carbon stock in the forest stages.
Data availability
The data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.10966030.
References
Acácio V, Holmgren M, Jansen PA, Schrotter O (2007) Multiple recruitment limitation causes arrested succession in mediterranean cork oak systems. Ecosystems 10:1220–1230. https://doi.org/10.1007/s10021-007-9089-9
Andrade BO, Boldrini II, Cadenazzi M et al (2019) Grassland vegetation sampling: a practical guide for sampling and data analysis. Acta Bot Bras 33:786–795. https://doi.org/10.1590/0102-33062019abb0160
Aryal DR, De Jong BHJ, Ochoa-Gaona S et al (2014) Carbon stocks and changes in tropical secondary forests of southern Mexico. Agr Ecosyst Environ 195:220–230. https://doi.org/10.1016/j.agee.2014.06.005
Badalamenti E, Battipaglia G, Gristina L, Novara A (2019) Carbon stock increases up to old growth forest along a secondary succession in Mediterranean island ecosystems. PLoS ONE 14:13. https://doi.org/10.1371/journal.pone.0220194
Bartholomée O, Grigulis K, Colace M-P et al (2018) Methodological uncertainties in estimating carbon storage in temperate forests and grasslands. Ecol Ind 95:331–342. https://doi.org/10.1016/j.ecolind.2018.07.054
Bastin J-F, Finegold Y, Garcia C et al (2019) The global tree restoration potential. Science 365:76–79. https://doi.org/10.1126/science.aax0848
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163. https://doi.org/10.1111/j.1365-2389.1996.tb01386.x
Bauters M, Vercleyen O, Vanlauwe B et al (2019) Long-term recovery of the functional community assembly and carbon pools in an African tropical forest succession. Biotropica 51:319–329. https://doi.org/10.1111/btp.12647
Benjamin K, Domon G, Bouchard A (2005) Vegetation composition and succession of abandoned farmland: effects of ecological, historical and spatial factors. Landscape Ecol 20:627–647. https://doi.org/10.1007/s10980-005-0068-2
Blanco J, Sourdril A, Deconchat M et al (2020) How farmers feel about trees: perceptions of ecosystem services and disservices associated with rural forests in southwestern France. Ecosyst Serv 42:101066. https://doi.org/10.1016/j.ecoser.2020.101066
Callot G, Bornand M, Favrot C (1966) Carte pédologique du val d’Allier
CEREMAC (2000) Les friches dans le Massif central. Mythes et réalités., CEREMAC. Presses Universitaires Blaise Pascal
Chang X, Chai Q, Wu G et al (2017) Soil organic carbon accumulation in abandoned croplands on the loess plateau: vegetation succession and SOC recovery. Land Degrad Dev 28:1519–1527. https://doi.org/10.1002/ldr.2679
Chazdon RL, Lindenmayer D, Guariguata MR et al (2020) Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ Res Lett 15:043002. https://doi.org/10.1088/1748-9326/ab79e6
Chojnacky D, Amacher M, Gavazzi M (2009) Separating duff and litter for improved mass and carbon estimates. South J Appl for 33:29–34. https://doi.org/10.1093/sjaf/33.1.29
Ciurzycki W, Marciszewska K, Zaniewski PT (2021) Formation of undergrowth species composition in birch forests on former farmland in the early stage of spontaneous secondary succession. Sylwan 165:392–401. https://doi.org/10.26202/sylwan.2021055
Commissariat général au développement durable (2019) EFESE: La séquestration du carbone par les écosystèmes français
Cramer V, Hobbs RJ (2007) Old Fields. Dynamic and restoration of abandoned farmland. Society for Ecological Restoration International. Island Press
Dantas de Miranda M, Pereira HM, Corley MFV, Merckx T (2019) Beta diversity patterns reveal positive effects of farmland abandonment on moth communities. Sci Rep 9:1549. https://doi.org/10.1038/s41598-018-38200-3
de Groot RS, Wilson MA, Boumans RMJ (2002) A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol Econ 41:393–408. https://doi.org/10.1016/S0921-8009(02)00089-7
de Jesus Silva CV, dos Santos JR, Galvao LS et al (2016) Floristic and structure of an Amazonian primary forest and a chronosequence of secondary succession. ACTA Amazon 46:133–150. https://doi.org/10.1590/1809-4392201504341
Dimobe K, Kuyah S, Dabré Z et al (2019) Diversity-carbon stock relationship across vegetation types in W National park in Burkina Faso. For Ecol Manage 438:243–254. https://doi.org/10.1016/j.foreco.2019.02.027
Facioni L, Burrascano S, Chiti T et al (2019) Changes in plant diversity and carbon stocks along a succession from semi-natural grassland to submediterranean Quercus cerris L. woodland in Central Italy. Phytocoenologia. https://doi.org/10.1127/phyto/2019/0299
Finzi AC, Giasson M, Barker Plotkin AA et al (2020) Carbon budget of the Harvard Forest Long-Term Ecological Research site: pattern, process, and response to global change. Ecol Monogr 90:e01423. https://doi.org/10.1002/ecm.1423
Fonderflick J, Caplat P, Lovaty F et al (2010) Avifauna trends following changes in a Mediterranean upland pastoral system. Agr Ecosyst Environ 137:337–347. https://doi.org/10.1016/j.agee.2010.03.004
Foote RL, Grogan P (2010) Soil carbon accumulation during temperate forest succession on abandoned low productivity agricultural lands. Ecosystems 13:795–812. https://doi.org/10.1007/s10021-010-9355-0
Freschet GT, Valverde-Barrantes OJ, Tucker CM et al (2017) Climate, soil and plant functional types as drivers of global fine-root trait variation. J Ecol 105:1182–1196. https://doi.org/10.1111/1365-2745.12769
Gerard F, Petit S, Smith G et al (2010) Land cover change in Europe between 1950 and 2000 determined employing aerial photography. Prog Phys Geogr Earth Environ 34:183–205. https://doi.org/10.1177/0309133309360141
Gillet F (1998) La phytosociologie synusiale intégrée
Gogoi A, Sahoo UK, Saikia H (2020) Vegetation and ecosystem carbon recovery following shifting cultivation in Mizoram-Manipur-Kachin rainforest eco-region. Southern Asia Ecol Process 9:21. https://doi.org/10.1186/s13717-020-00225-w
Gosheva S, Walthert L, Niklaus PA et al (2017) Reconstruction of historic forest cover changes indicates minor effects on carbon stocks in Swiss forest soils. Ecosystems 20:1512–1528. https://doi.org/10.1007/s10021-017-0129-9
Guidi C, Vesterdal L, Gianelle D, Rodeghiero M (2014) Changes in soil organic carbon and nitrogen following forest expansion on grassland in the Southern Alps. For Ecol Manage 328:103–116. https://doi.org/10.1016/j.foreco.2014.05.025
Haines-Young R, Potschin M (2018) Common international classification of ecosystem services (CICES) V5.1 and Guidance on the Application of the Revised Structure
Hardiman BS, Gough CM, Halperin A et al (2013) Maintaining high rates of carbon storage in old forests: a mechanism linking canopy structure to forest function. For Ecol Manage 298:111–119. https://doi.org/10.1016/j.foreco.2013.02.031
Harmer R, Peterken G, Kerr G, Poulton P (2001) Vegetation changes during 100 years of development of two secondary woodlands on abandoned arable land. Biol Conserv. https://doi.org/10.1016/S0006-3207(01)00072-6
Haut Conseil pour le Climat (2022) Dépasser les constats. Mettre en oeuvre les solutions
Hétier (1973) Carte pédologique de la chaîne des Puys
Holl KD, Zahawi RA (2014) Factors explaining variability in woody above-ground biomass accumulation in restored tropical forest. For Ecol Manage 319:36–43. https://doi.org/10.1016/j.foreco.2014.01.024
Holmes MA, Matlack GR (2018) Assembling the forest herb community after abandonment from agriculture: Long-term successional dynamics differ with land-use history. J Ecol 106:2121–2131. https://doi.org/10.1111/1365-2745.12970
Hong S, Yin G, Piao S et al (2020) Divergent responses of soil organic carbon to afforestation. Nat Sustain 3:694–700. https://doi.org/10.1038/s41893-020-0557-y
Hooker TD, Compton JE (2003) Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonment. Ecol Appl 13:299–313. https://doi.org/10.1890/1051-0761(2003)013[0299:FECANA]2.0.CO;2
IPCC (2022) Climate Change 2022: Impacts, Adaptation, and Vulnerability
IPCC (2006a) Guidelines for National Greenhouse Gas Inventories. Chapter 4 Forest Land
IPCC (2006b) Guidelines for National Greenhouse Gas Inventories. Chapter 6 Grasslands
Jackson RB, Lajtha K, Crow SE et al (2017) The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu Rev Ecol Evol Syst 48:419–445. https://doi.org/10.1146/annurev-ecolsys-112414-054234
Jafari M, Ansari-Pour N (2019) Why, when and how to adjust your p values? Cell J 20:604–607. https://doi.org/10.22074/cellj.2019.5992
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:14
Jones IL, DeWalt SJ, Lopez OR et al (2019) Above- and belowground carbon stocks are decoupled in secondary tropical forests and are positively related to forest age and soil nutrients respectively. Sci Total Environ 697:133987. https://doi.org/10.1016/j.scitotenv.2019.133987
Kalt G, Mayer A, Theurl MC et al (2019) Natural climate solutions versus bioenergy: Can carbon benefits of natural succession compete with bioenergy from short rotation coppice? GCB Bioenergy 11:1283–1297. https://doi.org/10.1111/gcbb.12626
Keith H, Lindenmayer D, Mackey B et al (2014) Managing temperate forests for carbon storage: impacts of logging versus forest protection on carbon stocks. Ecosphere 5:34. https://doi.org/10.1890/ES14-00051.1
Lasanta T, Nadal-Romero E, Arnáez J (2015) Managing abandoned farmland to control the impact of re-vegetation on the environment. The state of the art in Europe. Environ Sci Policy 52:99–109. https://doi.org/10.1016/j.envsci.2015.05.012
Lasanta T, Sánchez-Navarrete P, Medrano-Moreno L et al (2020) Soil quality and soil organic carbon storage in abandoned agricultural lands: effects of revegetation processes in a Mediterranean mid-mountain area. Land Degrad Dev 31:2830–2845. https://doi.org/10.1002/ldr.3655
Leal Filho W, Mandel M, Al-Amin AQ et al (2017) An assessment of the causes and consequences of agricultural land abandonment in Europe. Int J Sust Dev World 24:554–560. https://doi.org/10.1080/13504509.2016.1240113
Lecointe S, Nys C, Walter C et al (2006) Estimation of carbon stocks in a beech forest (Fougères Forest – W. France): extrapolation from the plots to the whole forest. Ann for Sci 63:139–148. https://doi.org/10.1051/forest:2005106
Lee S, Lee DK (2018) What is the proper way to apply the multiple comparison test? Korean J Anesthesiol 71:353–360. https://doi.org/10.4097/kja.d.18.00242
Legendre P, Legendre L (2012) Numerical Ecology. Elsevier, Third English Edition
Lepart J, Escarré J (1983) La succession végétale, mécanismes et modèles: analyse bibliographique. Bull D’écologie Paris 14:133–178
Li W, Li J, Liu S et al (2017) Magnitude of species diversity effect on aboveground plant biomass increases through successional time of abandoned farmlands on the eastern Tibetan Plateau of China. Land Degrad Develop 28:370–378. https://doi.org/10.1002/ldr.2607
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76. https://doi.org/10.1038/35083573
Lu D, Moran E, Mausel P (2002) Linking Amazonian secondary succession forest growth to soil properties. Land Degrad Dev 13:331–343. https://doi.org/10.1002/ldr.516
Lyytimaki J, Sipila M (2009) Hopping on one leg: the challenge of ecosystem disservices for urban green management. Urban for Urban Green 8:309–315. https://doi.org/10.1016/j.ufug.2009.09.003
Manrique LA, Jones CA, Dyke PT (1991) Predicting cation-exchange capacity from soil physical and chemical properties. Soil Sci Soc Am J 55:787–794. https://doi.org/10.2136/sssaj1991.03615995005500030026x
Måren IE, Sharma LN (2021) Seeing the wood for the trees: carbon storage and conservation in temperate forests of the Himalayas. For Ecol Manage 487:119010. https://doi.org/10.1016/j.foreco.2021.119010
Marín-Spiotta E, Sharma S (2013) Carbon storage in successional and plantation forest soils: a tropical analysis: carbon in reforested and plantation soils. Glob Ecol Biogeogr 22:105–117. https://doi.org/10.1111/j.1466-8238.2012.00788.x
Matos FAR, Magnago LFS, Aquila Chan Miranda C et al (2020) Secondary forest fragments offer important carbon and biodiversity cobenefits. Glob Change Biol 26:509–522. https://doi.org/10.1111/gcb.14824
Mayer PM (2008) Ecosystem and decomposer effects on litter dynamics along an old field to old-growth forest successional gradient. Acta Oecologica 33:222–230. https://doi.org/10.1016/j.actao.2007.11.001
Meraj G, Singh SK, Kanga S, Islam MdN (2022) Modeling on comparison of ecosystem services concepts, tools, methods and their ecological-economic implications: a review. Model Earth Syst Environ 8:15–34. https://doi.org/10.1007/s40808-021-01131-6
Meyfroidt P, Lambin EF (2011) Global forest transition: prospects for an end to deforestation. Annu Rev Environ Resour 36:343–371. https://doi.org/10.1146/annurev-environ-090710-143732
Assessment ME (ed) (2005) Ecosystems and human well-being : synthesis. Island Press, Washington, DC
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96. https://doi.org/10.1111/j.1365-2486.2005.001043.x
Montero G, Pasalodos-Tato M, López-Senespleda E, et al (2013) Ecuaciones para la estimación de la biomasa en matorrales y arbustedos mediterráneos. Congreso Forestal Español
Moran EF, Brondizio ES, Tucker JM (2000) Effects of soil fertility and land-use on forest succession in Amazônia. For Ecol Manage 139:93–108. https://doi.org/10.1016/S0378-1127(99)00337-0
Morel L, Barbe L, Jung V et al (2020) Passive rewilding may (also) restore phylogenetically rich and functionally resilient forest plant communities. Ecol Appl 30:e02007. https://doi.org/10.1002/eap.2007
Nunes AN (2019) Mudanças na paisagem e serviços dos ecossistemas: Abandono agrícola e variação no carbono orgânico dos solos. GeoJou. https://doi.org/10.14195/0871-1623_39_1
Palmer C, Markstein K, Tanner L et al (2019) Experimental test of temperature and moisture controls on the rate of microbial decomposition of soil organic matter: preliminary results. AIMS Geosci 5:886–898. https://doi.org/10.3934/geosci.2019.4.886
Pan Y, Birdsey RA, Fang J et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Peco B, Navarro E, Carmona CP et al (2017) Effects of grazing abandonment on soil multifunctionality: the role of plant functional traits. Agr Ecosyst Environ 249:215–225. https://doi.org/10.1016/j.agee.2017.08.013
Perpiña Castillo C, Jacobs-Crisioni C, Diogo V, Lavalle C (2021) Modelling agricultural land abandonment in a fine spatial resolution multi-level land-use model: an application for the EU. Environ Model Softw 136:104946. https://doi.org/10.1016/j.envsoft.2020.104946
Piché N, Kelting DL (2015) Recovery of soil productivity with forest succession on abandoned agricultural land: Soil recovery on former agricultural lands. Restor Ecol 23:645–654. https://doi.org/10.1111/rec.12241
Post WM, Kwon KC (2000) Soil carbon sequestration and land-use change: processes and potential. Glob Change Biol 6:317–327. https://doi.org/10.1046/j.1365-2486.2000.00308.x
Potapov P, Turubanova S, Hansen MC et al (2022) Global maps of cropland extent and change show accelerated cropland expansion in the twenty-first century. Nat Food 3:19–28. https://doi.org/10.1038/s43016-021-00429-z
Prach K, Walker LR (2011) Four opportunities for studies of ecological succession. Trends Ecol Evol 26:119–123. https://doi.org/10.1016/j.tree.2010.12.007
Raj KD, Mathews G, Bharath MS et al (2018) Climate change-induced coral bleaching in Malvan Marine Sanctuary, Maharashtra, India. Curr Sci 114:384–387. https://doi.org/10.18520/cs/v114/i02/384-387
Ramankutty N, Foley JA (1999) Estimating historical changes in global land cover: Croplands from 1700 to 1992. Global Biogeochem Cycles 13:997–1027. https://doi.org/10.1029/1999GB900046
Raunkiaer, (1934) The life forms of plants and statistical plant geography. Oxford University Press, London
Risch AC, Jurgensen MF, Page-Dumroese DS et al (2008) Long-term development of above- and below-ground carbon stocks following land-use change in subalpine ecosystems of the Swiss National Park. Can J for Res 38:1590–1602. https://doi.org/10.1139/X08-014
Robinson SJB, van den Berg E, Meirelles GS, Ostle N (2015) Factors influencing early secondary succession and ecosystem carbon stocks in Brazilian Atlantic Forest. Biodivers Conserv 24:2273–2291. https://doi.org/10.1007/s10531-015-0982-9
Romero-Díaz A, Ruiz-Sinoga JD, Robledano-Aymerich F et al (2017) Ecosystem responses to land abandonment in Western Mediterranean Mountains. CATENA 149:824–835. https://doi.org/10.1016/j.catena.2016.08.013
Roux C (2017) De la Limagne à la chaîne des Puys. Approche analytique intégrative pour l’étude des végétations actuelles et potentielles en moyenne montagne tempérée. Université Clermont Auvergne
Rudel TK, Coomes OT, Moran E et al (2005) Forest transitions: towards a global understanding of land use change. Glob Environ Chang 15:23–31. https://doi.org/10.1016/j.gloenvcha.2004.11.001
Rühl J, Schnittler M (2011) An empirical test of neighbourhood effect and safe-site effect in abandoned Mediterranean vineyards. Acta Oecologica 37:71–78. https://doi.org/10.1016/j.actao.2010.11.009
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158. https://doi.org/10.1007/s11104-010-0391-5
Sharma CM, Gairola S, Baduni NP et al (2011) Variation in carbon stocks on different slope aspects in seven major forest types of temperate region of Garhwal Himalaya, India. J Biosci 36:701–708. https://doi.org/10.1007/s12038-011-9103-4
Shi S, Zhang W, Zhang P et al (2013) A synthesis of change in deep soil organic carbon stores with afforestation of agricultural soils. For Ecol Manage 296:53–63. https://doi.org/10.1016/j.foreco.2013.01.026
Sierra CA, Trumbore SE, Davidson EA et al (2015) Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J Adv Model Earth Syst 7:335–356. https://doi.org/10.1002/2014MS000358
Smith P, Soussana J, Angers D et al (2020) How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob Change Biol 26:219–241. https://doi.org/10.1111/gcb.14815
Stoate C, Báldi A, Beja P et al (2009) Ecological impacts of early 21st century agricultural change in Europe: a review. J Environ Manage 91:22–46. https://doi.org/10.1016/j.jenvman.2009.07.005
Thibault M, Thiffault E, Bergeron Y et al (2022) Afforestation of abandoned agricultural lands for carbon sequestration: how does it compare with natural succession? Plant Soil 475:605–621. https://doi.org/10.1007/s11104-022-05396-3
Tison J-M, Foucault B de (2014) Flora Gallica : Flore de France. Biotope Editions, Mèze
Ustaoglu E, Collier MJ (2018) Farmland abandonment in Europe: an overview of drivers, consequences, and assessment of the sustainability implications. Environ Rev 26:396–416. https://doi.org/10.1139/er-2018-0001
Vacek Z, Vacek S, Cukor J (2023) European forests under global climate change: review of tree growth processes, crises and management strategies. J Environ Manage 332:117353. https://doi.org/10.1016/j.jenvman.2023.117353
Vallet P, Meredieu C, Seynave I et al (2009) Species substitution for carbon storage: Sessile oak versus Corsican pine in France as a case study. For Ecol Manage 257:1314–1323. https://doi.org/10.1016/j.foreco.2008.11.034
van Vliet J, de Groot HLF, Rietveld P, Verburg PH (2015) Manifestations and underlying drivers of agricultural land use change in Europe. Landsc Urban Plan 133:24–36. https://doi.org/10.1016/j.landurbplan.2014.09.001
Velázquez E, Martínez-Jaraíz C, Wheeler C et al (2022) Forest expansion in abandoned agricultural lands has limited effect to offset carbon emissions from Central-North Spain. Reg Environ Change 22:132. https://doi.org/10.1007/s10113-022-01978-0
Vesterdal L, Clarke N, Sigurdsson BD, Gundersen P (2013) Do tree species influence soil carbon stocks in temperate and boreal forests? For Ecol Manage 309:4–18. https://doi.org/10.1016/j.foreco.2013.01.017
Vigerstol KL, Aukema JE (2011) A comparison of tools for modeling freshwater ecosystem services. J Environ Manage 92:2403–2409. https://doi.org/10.1016/j.jenvman.2011.06.040
Vilà-Cabrera A, Espelta JM, Vayreda J, Pino J (2017) “New Forests” from the twentieth century are a relevant contribution for C storage in the Iberian Peninsula. Ecosystems 20:130–143. https://doi.org/10.1007/s10021-016-0019-6
Villamagna AM, Angermeier PL, Bennett EM (2013) Capacity, pressure, demand, and flow: a conceptual framework for analyzing ecosystem service provision and delivery. Ecol Complex 15:114–121. https://doi.org/10.1016/j.ecocom.2013.07.004
von Döhren P, Haase D (2015) Ecosystem disservices research: a review of the state of the art with a focus on cities. Ecol Ind 52:490–497. https://doi.org/10.1016/j.ecolind.2014.12.027
Walker LR, Wardle DA, Bardgett RD, Clarkson BD (2010) The use of chronosequences in studies of ecological succession and soil development: chronosequences, succession and soil development. J Ecol 98:725–736. https://doi.org/10.1111/j.1365-2745.2010.01664.x
Weissgerber M, Chanteloup L, Bonis A (2022) Land-use and cover changes in the Massif Central region of France. Extent of afforestation and its consequences on ecosystems, Paris
Wendt JW, Hauser S (2013) An equivalent soil mass procedure for monitoring soil organic carbon in multiple soil layers. European J Soil Science 64:58–65. https://doi.org/10.1111/ejss.12002
Yan J, Wang Y, Zhou G, Zhang D (2006) Estimates of soil respiration and net primary production of three forests at different succession stages in South China: estimates of soil respiration and NPP. Glob Change Biol 12:810–821. https://doi.org/10.1111/j.1365-2486.2006.01141.x
Yesilonis I, Szlavecz K, Pouyat R et al (2016) Historical land use and stand age effects on forest soil properties in the Mid-Atlantic US. For Ecol Manage 370:83–92. https://doi.org/10.1016/j.foreco.2016.03.046
Yue J-W, Guan J-H, Deng L et al (2018) Allocation pattern and accumulation potential of carbon stock in natural spruce forests in northwest China. PeerJ 6:e4859. https://doi.org/10.7717/peerj.4859
Zianis D, Mencuccini M (2004) On simplifying allometric analyses of forest biomass. For Ecol Manage 187:311–332. https://doi.org/10.1016/j.foreco.2003.07.007
Ziegler AD, Phelps J, Yuen JQ et al (2012) Carbon outcomes of major land-cover transitions in SE Asia: great uncertainties and REDD + policy implications. Glob Change Biol 18:3087–3099. https://doi.org/10.1111/j.1365-2486.2012.02747.x
Zornoza R, Mataix-Solera J, Guerrero C et al (2009) Comparison of soil physical, chemical, and biochemical properties among native forest, maintained and abandoned almond orchards in mountainous areas of Eastern Spain. Arid Land Res Manag 23:267–282. https://doi.org/10.1080/15324980903231868
Acknowledgements
This research work was funded by the Regional Council of Auvergne Rhône Alpes, through the ATTRIHUM axis of the CPER. The authors are grateful to the anonymous reviewers for their constructive comments and to Annie Buchwalter for correcting written English. They would like to thank Luna Chambaud and Simon Faure for their help in data collection. The first author is thankful to Benjamin Allard and Julie Crabot for their helpful insights.
Funding
Open Access funding enabled and organized by Projekt DEAL. The funding was provided by Région Auvergne-Rhône-Alpes Region Council of Auvergne Rhône Alpes, through the ATTRIHUM axis of the CPER (Grant No. 1701429401-61617).
Author information
Authors and Affiliations
Contributions
MW conducted the surveys and reviews of existing research. MW and AB worked on methods. MW conducted data collection, results analysis, wrote the main manuscript text and prepared figures. AB and LC supervised the project and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review by any other publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weissgerber, M., Chanteloup, L. & Bonis, A. Carbon stock increase during post-agricultural succession in central France: no change of the superficial soil stock and high variability within forest stages. New Forests (2024). https://doi.org/10.1007/s11056-024-10044-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11056-024-10044-y