Abstract
In degraded forest ecosystems, reintroduction of keystone-woody species is an important step for restoration because it provides regeneration niches. However, lack of information on how to propagate species restricts the use of native species; specially in tropical dry forests where seed germination is seasonal and is synchronized with the onset of the wet season. We evaluated the dormancy-breaking and germination requirements of nine keystone-woody species from Dry Chaco Forest. Most fresh viable seeds of the keystone species are nondormant, but viability decreases during dry-cold storage restricting the use of some species. Seeds of three keystone species germinated to higher percentages in light than darkness and those of five species germinated equally well in light and darkness; seeds of Castela coccinea germinated to higher percentages in darkness than in light. Alternating vs. constant temperatures had no effect on germination in seven species. Seeds of Anisocapparis speciosa and Cynophalla retusa were nondormant and remained viable for only 1 month during cold-dry storage; and 62–95% of the seeds of the other seven species were nondormant. Dormancy-break was studied in five of the seven species; seeds of four species had physiological dormancy and one had physical dormancy. Dormancy-breaking was promoted by environmental conditions in the habitat, i.e. warm stratification, after seed dispersal. Castela coccinea, Achatocarpus praecox, Mimosa detinens, and Capparicordis tweedieana are the most suitable keystone species for the restoration of overgrazed areas in the Dry Chaco Forest because their seeds germinate to a high percentage and retain viability during dry storage at low temperatures. Seeds of Mimosa detinens and Capparicordis tweedieana required mechanical scarification and 6-weeks of warm stratification, respectively, for dormancy-break.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In extremely degraded forest or shrubland ecosystems, reintroduction of keystone-woody species can be an important step for restoration (Aronson et al. 1993; Khurana and Singh 2001; Elliott et al. 2002; Brooker et al. 2008; Pérez et al. 2019). Keystone species are species critical to ecosystem function and structure, and when established they accelerate secondary succession by providing regeneration niches for other species (Rey-Benayas et al. 2008; McDonald et al. 2016; Navarro-Cano et al. 2019). Nurse plant species are highly desirable as keystone species because they can facilitate the establishment, survival, and growth of seedlings (Bertness and Callaway 1994; Flores and Jurado 2003; Padilla and Pugnaire 2006; Gómez-Aparicio 2009), so they are recommended for restoration programs. In semiarid lands, many studies have shown that seedling survival of herbs, trees, and shrubs is higher under the shade of shrubs than in open spaces (Lieberman and Li 1992; Ray and Brown 1995; Breshears et al. 1998; Holl 2002; Castro et al. 2004; Gómez-Aparicio et al. 2004; Barchuk et al. 2008; Gómez-Aparicio 2009). Furthermore, keystone species attract animals that disperse seeds, since these species provide bird-perching structures and animal refugees (Callaway 1995; Holl 2002; Flores and Jurado 2003; Tálamo et al. 2015b). Choosing mid-successional shrub species as keystone species is highly desirable because they can become established in the harsh environmental conditions of degraded places and grow faster than late-successional species (Padilla-Ruiz et al. 2004; Padilla et al. 2009; Martínez-Carretero and Dalmasso 2015; Waiboonya and Elliott 2020). However, before a potentially good shrub-nurse plant species can be used as a keystone species in restoration programs we must be able to propagate it. Unfortunately, for many ecosystems information on how to germinate the seeds of native species that would be good nurse plants and keystone species is not available (Elliott et al. 2003; Meli et al. 2014).
In tropical dry forests, seed germination is restricted to periods with favorable soil moisture conditions and temperatures (Khurana and Singh 2001); thus, seedlings have time to grow and develop a good root system before the onset of the dry season (Garwood 1983; Khurana and Singh 2001; Jurado and Flores 2005; Baskin and Baskin 2014; Waiboonya and Elliott 2020). Plant species have developed two strategies to synchronize seed germination with the occurrence of the favorable wet season: (1) dispersing non-dormant seeds at the beginning of the rainy season and (2) delaying germination until the next rainy season via seed dormancy (Lieberman and Li 1992; van Schaik et al. 1993). Both strategies have been reported to occur simultaneously among the species in different seasonal tropical forests such as those on Barro Colorado Island in Panamá (Garwood 1983), in Pinkwae in Ghana (Lieberman and Li 1992), the canal watershed in Panamá (Sautu et al. 2006), central Brazil (Salazar et al. 2011), and the Cerrado of Brazil (Ramos et al. 2017; Escobar et al. 2018). Thus, it is likely that these strategies are present in other seasonal tropical forests that have not been studied, such as Dry Chaco Forest. Therefore, we hypothesized that seeds maturing at the beginning of the rainy season would be nondormant, and those maturing at the end of the rainy season would be dormant. To test this hypothesis, we tested fresh seeds of each species to determine if they were dormant or nondormant at the moment of seed dispersal.
In shrubs of dry tropical forests (tropical deciduous forest sensu Baskin and Baskin 2014) about 80% of the species have dormant seeds with physical dormancy followed by physiological dormancy in importance; while morphophysiological dormancy is not common (Baskin and Baskin 2014). If dormant seeds are dispersed when environmental conditions are not favorable for germination (i.e. too cold and/or dry), the environmental conditions during this unfavorable period for seedling growth may promote the breaking of dormancy. For example, physiological dormancy (PD) can be broken during the dry season in tropical regions, and seeds germinate as soon as it rains (Baskin and Baskin 2014, Baskin and Baskin 2022). However, some seeds with PD of various species from dry tropical forests in Brazil required 47 to 113 days for initiation of germination on a moist substrate (Zamith and Scarano 2004); while seeds of Adina cordifolia in India germinated over a 7-week period (Beniwal and Singh 1989). In species with physical dormancy (PY), i.e. a water-impermeable seed/fruit coat, a period of warm dry weather can make the seeds sensitive to dormancy-breaking conditions (e.g. wet soil) that follow the time when seeds became sensitive (e.g. Gama-Arachchige et al. 2012). That is, the breaking of PY is a two-step process, and the first step can occur when it is too dry in the habitat for germination and seedling establishment to occur; the second step occurs after the rainy season begins. Thus, we hypothesized that dormancy-break would be promoted by the environmental conditions that occur after the seeds are dispersed. To test this hypothesis, we determined the dormancy-breaking requirements of dormant seeds and compared them to environmental factors in the habitat following seed dispersal.
After dormancy is broken, the germination at the beginning of the favorable (wet) season gives seedlings the maximum period of time for establishment and growth before the onset of the dry season (Khurana and Singh 2001; Jurado and Flores 2005; Baskin and Baskin 2014); therefore, the climate conditions during that time would enhance germination if we germinate seeds in controlled conditions. In tropical dry forests, the optimum temperature for germination of seeds of 60 species of shrubs was 26.9 ± 0.2 °C, but data for germination in light vs. dark were available for only six species, which germinated equally well in light and dark (Baskin and Baskin 2014). Understanding the requirements for dormancy-break and germination is the first step to facilitate seedling propagation in nurseries for restoration activities.
Seed storage is often an important part of a restoration program in which plants are propagated from seeds (De Vitis et al. 2020); and several studies pointed out the necessity of storing seeds, for example for seeds of Carex spp. from meadow wetlands in United States (Budelsky and Galatowitsch 1999), seeds of trees from a tropical dry forest in Mexico (Cervantes et al. 2014), and seeds of Mimosa foliolosa from a seasonal grassland in Brazil (Silveira et al. 2014). Storage in cool dry conditions, e.g. at 4–5 °C in a refrigerator may be a convenient way for restoration workers to store small seed lots (Baskin and Baskin 2014). Since seeds of many tropical dry forest tree species lose viability during dry cold storage or even at room temperatures (Khurana and Singh 2001), it is important to know how long seeds will remain viable during dry storage.
Large areas of dry tropical forests have been destroyed and need to be restored (Hansen et al. 2013). In South America, Dry Chaco Forest occupies 1,100,000 Km2 in Argentina, Bolivia, Brazil, and Paraguay (Kuemmerle et al. 2017), while the majority is in Argentina (600,000 Km2) (Morello and Rodriguez 2009). Rapid loss of Dry Chaco Forest is occurring in northwestern Argentina. From 1992 to 1999, annual loss of this type of forest in Argentina was 5% (Boletta et al. 2006), but between 1972 and 2007 deforestation reached 43% (Gasparri and Grau 2009). Agricultural expansion, mainly for soybean cultivation, has changed this area into crop fields, resulting in fragmentation of the forest into small remnants (Zak et al. 2004; Gasparri and Grau 2009). In 2004, the conversion of Argentinean Dry Chaco Forest into agricultural land was 91,000 km2 (Morello and Rodriguez 2009). In the forest remnants, extensive grazing is practiced transforming these areas into patches of highly degraded secondary forest. Natural regeneration of woody species is decreased because livestock diminish propagule sources (due to selective grazing), reduce the emergence of seedlings (due to trampling), and decrease regeneration niches (due to soil compaction, erosion, and loss of soil nutrients) (Abril and Bucher 1999; Czeglédi and Radácsi 2005; Macchi and Grau 2012; Mazzini et al. 2018). Reintroduction of keystone-nurse-woody species could be a suitable restoration technique for Dry Chaco Forest because facilitation is an important mechanism for the establishment of some species (Páez and Marco 2000; Barchuk et al. 2008). Further, the use of thorny shrubs as keystone species would enhance their ability to serve as a refuge for the establishment of seedlings (Tálamo et al. 2015a). However, if we want to propagate keystone-woody species to restore overgrazed sites knowledge of seed dormancy-breaking and germination requirements is a key first step. The objective of our research was to determine the dormancy-breaking and germination requirements of nine shrub species from Dry Chaco Forest that potentially could perform as keystone-nurse-woody species. Specifically, we determined the (1) kind/class of dormancy, (2) effective treatments to break dormancy, (3) light and temperature requirements for germination of nondormant seeds and (4) the retention of seed viability during dry storage at 4 °C.
Methodology
Study species
Nine native shrubs species of Dry Chaco Forest were selected (Table 1). They could be suitable keystone species for the restoration of overgrazed sites since they have a conspicuous evergreen canopy, functional characteristics for grazing deterrence (thorns or chemical compounds), and fruits that attract native seed-dispersing animals.
Seed collection
Fruits were obtained from Copo National Park in Santiago del Estero, Argentina (25° 39′ 11″ to 26° 10′ 37″ S and 61° 42′ 46″ to 62° 12′ 55″ W). Vegetation in the park corresponds to Dry Chaco Forest, semi-deciduous thorn forest (Bucher 1982), or dry tropical forest (Walter and Burnett 1971). The climate is semiarid and seasonal (Bucher 1982). The warm-rainy season (favorable period for seed germination) occurs between October and March; during which time the mean temperature is 25.6 °C, and the daily mean maximum and minimum temperatures are 32.4 °C during the day and 19.2 °C during the night. During the wet season, precipitation is 649.35 mm. The cold-dry season (unfavorable period for seed germination) occurs between April to September; during which time the mean temperature is 18.1 °C, and the daily mean maximum and minimum temperatures are 25.0 °C during the day and 11.6 °C during the night. During the dry season, precipitation is only 208.7 mm, and the water balance is negative resulting in soil moisture deficit (average of the last 15 years of the closest climate stations located in Sáenz Peña, Castelli, and Pampa del Infierno in Argentina) (INTA 2020).
Ripe fruits were collected from a minimum of 20 adult plants of each species between 2016 and 2018. Seeds were removed from the dry fruits and berries, and the exocarp and mesocarp were removed from drupes, leaving the embryo enclosed by the endocarp. Seeds from berries and drupes (with the exocarp and mesocarp removed) were washed with tap water and dried at room temperature for 3 days. All seeds and drupes (hereafter drupes are referred to as seeds) were stored dry in closed plastic containers at 4 °C until used in experiments.
Germination test of fresh seeds
Four replicates of 25 freshly-harvested (3 to 7 days after collection) seeds of Castela coccinea, Capparicordis tweedieana, Capparis salicifolia, Anisocapparis speciosa, Achatocarpus praecox, Celtis pallida, Cynophalla retusa, Atamisquea emarginata, and Mimosa detinens were incubated at 27 °C in light and in darkness for 30 days following a randomized design. Seeds were placed in Petri dishes on filter paper moistened with distilled water. Seeds incubated in light were exposed to a 12 h daily photoperiod, while those incubated in darkness were covered with two layers of aluminum foil and placed in a black plastic bag. Germination in light was recorded at 2-day intervals (germination criterion was radicle emergence > 2 mm), while dark germination was recorded only at the end of the test. Non-germinated seeds were checked for viability using the cut test, and seeds with a white firm embryo were recorded as viable and those with a soft grey embryo as nonviable. Differences between treatments were tested using a t-test.
Seeds of A. speciosa and C. retusa (Capparaceae) were covered with fungi after 1 month of dry storage at 4 °C. Thus, due to their low viability we could not performed any additional experiments with seeds of these two species.
To determine the mechanism that controls seed germination, we compared the maximum percentage of germination and percentage of non-germinated viable seeds (i.e. dormant seeds) of each species with the month(s) of seed dispersal, using regression analyses.
Effect of constant versus alternating temperature on germination
We used seeds that had been stored dry at 4 °C for a period of time equal to that of the time between seed dispersal and the beginning of the following favorable wet period for seed germination: C. coccinea, 46 weeks; C. tweedieana, 44 weeks; C. salicifolia, 37 weeks; A. emarginata, 37 weeks; A. praecox, 34 weeks; C. pallida, 36 weeks; and M. detinens, 36 weeks. Seeds of all species were incubated in light (12 h daily photoperiod), except C. coccinea, at a constant temperature of 27 °C and at an alternating temperature regime of 35/20 °C. Seeds of C. coccinea were incubated in darkness because their germination percentage is higher in darkness than in light. Four replicates of 25 seeds were sown on the surface of white quartz sand moistened with distilled water in Petri dishes, and then we followed the procedure described for the germination test of fresh seeds. To determinate the effect on temperature on seed germination and to avoid any effect due to seed storage, we compared these results with the maximum germination obtained in the germination test of fresh seeds (seeds collected the same year). However, results for C. coccinea were compared with germination of fresh seeds and of stored seeds for 31 months, which were collected the previous year and tested following the procedure described for the germination test of fresh seeds.
To determine the effect of dry storage at 4 °C on retention of seed viability, we compared the viability of fresh and stored seeds of C. coccinea, C. tweedieana, C. salicifolia, A. praecox, C. pallida, A. emarginata, and M. detinens. Viable seeds were the germinated plus the non-germinated viable seeds.
Differences between treatments were tested using a one-way ANOVA. Variance homogeneity was tested using the Levenne test, and contrast between pairs of treatments was made using a Tukey test. When heteroscedasticity existed, we used a Kruskal–Wallis test.
Kind of dormancy and treatments to break dormancy
Baskin and Baskin (2004) defined five kinds (classes) of seed dormancy: morphological dormancy (MD), physical dormancy (PY), physiological dormancy (PD), morphophysiological dormancy (MPD), and combinational (PY + PD). From results of the germination test of fresh seeds, we determined that 23–39% of the fresh seeds of A. emarginata, C. salicifolia, C. tweedieana, C. pallida, and M. detinens was dormant; the other portion was non-dormant (see below). We determined the class of dormancy for the portion of dormant seeds of each species and tested different methods to break it.
Morphological dormancy
The embryo is differentiated (i.e. it has organs) but underdeveloped (small in comparison to the length of the seed), and it must growth inside the seed before germinates occurs (Baskin and Baskin 2014). To check for the presence of an underdeveloped embryo, three seeds of each species were cut opened, and embryo and seed length were measured. None of the five species had an underdeveloped embryo (See information about embryo morphology in Table 1).
Physical dormancy
Physical dormancy (PY) is caused by presence of a layer of water-impermeable palisade cells in the seed or fruit coat, while the embryo is fully developed and ready to germinate (Baskin and Baskin 2014). To determinate if seeds of C. tweedieana, C. salicifolia, C. pallida, A. emarginata, and M. detinens have PY, they were tested for imbibition of water. Twenty-five nontreated (intact-fresh) and manually scarified (cut individually with a razor blade) seeds were placed on filter paper moistened with distilled water. Seeds were weighed (to the nearest 0.0001 g) individually at time 0, and after 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 24 h, and 48 h. Each seed was removed from the Petri dish, blotted dry, and then weighed. Percentage increase in mass was calculated for each weighing time for each seed. Based on percentage of water imbibition, we decided if the seeds had a water-impermeable seed coat or not.
Fresh seeds of M. detinens collected in 2018 had PY, and they were given various treatments known to break PY: mechanical scarification (cutting a small hole at the cotyledon end of the seed with a razor blade), wet heat (dipping seeds in boiling water for 5 s, 15 s, and 5 min), wet (soaking in water for 24 h), soaking in concentrated sulfuric acid (for 5 min, 30 min, and 30 min). After performing the treatments, the seeds were tested for germination following the procedure for the germination test of fresh seeds. Differences between treatments were tested using a t-test to compare two treatments and a one-way ANOVA to compare more than two treatments. For ANOVA, variance homogeneity was tested using the Levenne test, and contrast between pairs of treatments was made using a Tukey test.
Physiological dormancy
Physiological dormancy (PD) is caused by a germination inhibiting mechanism in the embryo. Fresh seeds have a fully developed embryo, imbibe water but fail to germinate within 4 weeks (Baskin and Baskin 2014). Some seeds of C. pallida, C. tweedieana, A. emarginata, and C. salicifolia had PD.
Fresh seeds of Celtis pallida collected in 2017 and 2018 were given a GA3-treatment (0 ppm, 26 ppm, 260 ppm, or 2600 ppm of GA3), cold stratification (seeds placed in wet sand at 4 °C for 0, 3, 6, 9 and 12 weeks), and warm stratification (seeds placed in wet sand at 30 °C for 0, 3, 6, 9 and 12 weeks). Then, seeds were incubated at 27 °C for 30 days following the procedure for the germination test of fresh seeds.
Fresh seeds of Capparicordis tweedieana, C. salicifolia, and A. emarginata collected in 2017 and 2018 were given a GA3-treatment, cold stratification, warm stratification, and allowed to after-ripen at room temperature in paper bags for 0, 3, 6, 9 and 12 weeks. Then, seeds were incubated at 27 °C for 30 days following the procedure for the germination test of fresh seeds.
Differences between treatments were tested using one-way ANOVA; except when we analyzed the results for GA3 when we used a two-way ANOVA (factors GA3 + seed scarification). For ANOVA, variance homogeneity was tested using the Levenne test, and contrast between pairs of treatments was made using a Tukey test. When heteroscedasticity existed, we used a Kruskal–Wallis test.
Morphophysiological dormancy
Seeds have an underdeveloped embryo that also has PD. Seeds will not germinate until the PD has been broken and the embryo has grown inside the seed. Embryo growth may occur at the same time PD is being broken or after PD has been broken, depending on the species (Baskin and Baskin 2014). None of the five species had MPD.
Combinational dormancy
Seeds have a water-impermeable seed or fruit coat, and the embryo is fully developed but has PD. Depending on the species, PD may be broken before or after PY is broken (Baskin and Baskin 2014). None of the five species had PY + PD.
Data analyses
All tests were performed at a 5% of significance level. We used the statistical software INFOSTAT and R programming environment (R Core Team 2018) to create the figures.
Results
Germination test of fresh seeds
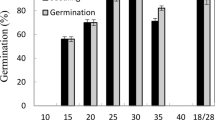
Based on number of viable seeds, maximum germination of A. speciosa, C. retusa, A. praecox, C. coccinea, A. emarginata, C. salicifolia, C. tweedieana, C. pallida, and M. detinens was 100.0%, 100%, 94.5%, 90.3%, 77.1%, 70.1%, 69.5%, 67.3%, and 62.1%, respectively (Fig. 1). Thus, all seeds of A. speciosa and C. retusa were nondormant, and 62 to 95% of the seeds of the other seven species were nondormant.
Germination of fresh seeds of keystone-woody species from Dry Chaco Forest. Percentage of seed germination (mean ± standard error). Seeds incubated at 27 °C in light (12 h of daily photoperiod) or in darkness for 30 days. Results of t-test (t-values, p-values). * indicates the significantly best treatment at a p-value < 0.05
Incubating seeds of C. coccinea in darkness increased germination by 29% compared to that of seeds in light, while incubating seeds of C. retusa, C. pallida, and C. salicifolia in light increased germination by 26%, 18%, and 17%, respectively, compared to that in darkness. Seeds of A. speciosa, C. tweedieana, A. praecox, A. emarginata, and M. detinens, were indifferent to light vs. dark (Fig. 1). After incubation in light, there were no non-germinated viable seeds of C. retusa and A. speciosa (Online Resource 1). Performance of the non-germinated seeds for each species is presented in Online Resource 1.
When we compared the highest germination percentage and the percentage of non-germinated viable seeds (Fig. 1 and Onlince Resource 1) of species that dispersed seeds at different times during the rainy season (Table1), the relationship was not linear nor significant (b = − 2.89, p = 0.5361), and in the intermediate months (December, January, and February) the relationship was positive. The percentage of viable seeds increased slightly towards the end of the rainy season, although the relationship was not linear nor significant (b = 1.73, p = 0.5792).
Effect of constant versus alternating temperature on germination
The alternating temperature regime (35/20 °C) did not increase the germination percentage of cool-dry-stored seeds of C. tweedieana, C. salicifolia, A. praecox, C. pallida, or M. detinens compared to constant temperature (27 °C) or compared to germination of fresh seeds at 27 °C. Fresh seeds of A. emarginata germinated to a higher percentage at 27 °C than those stored for 37 weeks and tested at 27 °C or 35/20 °C (Fig. 2). Performance of the non-germinated seeds for each species is presented in Online Resource 1.
Effect of constant vs. alternating temperature on germination of keystone-woody species from Dry Chaco Forest. Percentage of seed germination (mean ± standard error). Fresh seeds and stored seeds (dry at 4 °C) incubated at 27 °C or 35/20 °C in light (12 h of daily photoperiod) for 30 days. Results of ANOVA or Kruskal–Wallis (F-values or H-values, p-values). Different letters indicate significant differences at a p-value < 0.05 according to Tukey or Kruskal–Wallis test. w = weeks
Germination percentage of fresh seeds of C. coccinea tested in darkness at 27 °C did not differ from that of seeds dry-stored at 4 °C for 46 weeks and tested in light or darkness at 27 °C or 35/20 °C or from that of seeds dry-stored at 4 °C for 31 months and tested in light at 27 °C (Fig. 3). Performance of the non-germinated seeds is presented in Online Resource 1.
Effect of constant vs. alternating temperature on germination of Castela coccinea. Percentage of seed germination (mean ± standard error). Fresh seeds and stored seeds incubated at 27 °C or 35/20 °C in light (12 h of daily photoperiod) or darkness for 30 days. Result of ANOVA test (F-values, p-values). Different letters indicate significant differences at a p-value < 0.05 according to Tukey test. w = weeks, m = months
Dry storage at 4 °C promoted the retention of seed viability of C. coccinea, C. tweedieana, C. pallida, and A. praecox. However, dry storage at 4 °C decreased the percentage of viable seeds by 43% for C. salicifolia and 39.5% for A. emarginata and increased the percentage of viable seeds for M. detinens by 18% (Fig. 4).
Effect of dry storage at 4 °C on retention of seed viability of keystone-woody species from Dry Chaco Forest. Percentage of viable seeds of fresh seeds and stored seeds. Results of ANOVA or Kruskal–Wallis (F-values or H-values, p-values). Different letters indicate significant differences at a p-value < 0.05 according to Tukey or Kruskal–Wallis test. w = weeks, m=months
Kind of dormancy and treatments to break dormancy
Physical dormancy
Scarified and intact seeds of M. detinens had an increase in mass of 90% and 39% respectively. Mass increase for scarified and nonscarified seeds of C. tweedieana, C. salicifolia, C. pallida, and A. emarginata was similar and varied between 30–50% (Fig. 5).
Mechanical scarification, soaking in concentrated sulfuric acid (regardless of soaking time), and soaking in water for 24 h (wet treatment) improved germination of M. detinens by 54% (t = − 10.09, p = 0.0001), 39.6% (F = 34.70, p < 0.0001), and 14% (t = − 2.50, p = 0.0465), respectively. The best treatment was mechanical scarification, which resulted in 73.0 ± 3.4% germination. Wet heat treatment did not improve germination (F = 1.09, p = 0.3902) (Online Resource 2).
Physiological dormancy
The effect of GA3 on germination of C. pallida seeds depended on scarification (FGA3*esc = 6.41, p = 0.0024). If seeds were manually scarified, the germination percentage was low, and GA3 did not promote germination. In contrast, if seeds were not manually scarified germination percentage decreased at the highest GA3 concentration tested. Neither cold stratification nor warm stratification improved germination (F = 7.39, p = 0.0252; F = 7.20, p = 0.0019; respectively) (Online Resource 3).
The effect of GA3 on germination of C. tweediana seeds depended on scarification (FGA3*esc = 8.86, p = 0.0004). If seeds were manually scarified, the germination percentage decreased with increased GA3 concentration. In contrast, if seeds were not manually scarified the germination was similar to that of control seeds. Six weeks of warm stratification improved germination by 18.0% (H = 13.04, p = 0.0106), which was the best PD dormancy-breaking treatment resulting in 81.0 ± 1.7% germination. Three weeks of cold stratification did not have an effect on germination, and an increase in time of cold stratification decreased germination (F = 12.89, p = 0.0001). Afterripening decreased germination (F = 2.70, p = 0.0712) (Online Resource 4).
Three weeks of warm stratification of C. salicifolia seeds improved germination by 7.0% (F = 4.81, p = 0.0107), resulting in 12.0 ± 1.6% germination. Neither GA3 (including scarification treatment), cold stratification, nor afterripening improved germination (H = 7.31, p = 0.1066; H = 13.35, p = 0.0050; F = 71.24, p < 0.0001; respectively). While there was little change in number of viable seeds (compared to control) following treatment of non-scarified seeds with GA3, cold stratification, and afterripening at room temperature, only 1.0 ± 1.0% of the seeds receiving warm stratification were viable after 12 weeks (Online Resource 5).
Nine weeks of warm stratification of A. emarginata seeds improved germination by 7.0% (F = 34.71, p < 0.0001), resulting in 41.3 ± 0.4% germination; but a longer time of warm stratification diminished germination. Neither GA3 (including scarification treatment), cold stratification, nor afterripening (H = 11.34, p = 0.1182; F = 1.28, p = 0.3247; F = 2.22, p = 0.1156; respectively) improved germination (Online Resource 6).
Discussion
Our hypothesis that seeds maturing at the beginning of the rainy season will be nondormant and those maturing at the end of the rainy season will be dormant was not supported by our data. A high percentage (62–100%) of fresh viable seeds of all nine species was nondormant, regardless of when they were dispersed. Thus, a significant portion of the seed crop of the nine species can germinate (under good conditions) as soon as they are dispersed. However, for seven species, 6–39% of the seeds was dormant. Having some seeds that can germinate right away and other seeds that are dormant seems like a good strategy for survival in Dry Chaco Forest, which has a strong seasonality (Bianchi and Yáñez 1992). Perhaps, having dormancy and nondormant seeds at the time of dispersal is a bet hedging strategy for Chaco keystone species, but this needs to be tested experimentally to determine if a decrease in arithmetic mean fitness (i.e. seed dormancy) reduced variance in fitness and increases geometric mean fitness of the population. Nonetheless, the dormant portion of the seed cohort of the seven species can form at least a short-term soil seed bank that helps ensure persistence of the species in the Dry Chaco Forest. A similar strategy has been found in the arid zone of eastern Australia, where the germination response of eight species was studied. Fresh seeds of six species germinated rapidly in response to rainfall and those of two species had PD. Also, non-dormant seeds of many of these species had long viability, which helps to explain how they persisted in the soil seed bank (Duncan et al. 2019).
Although many seeds of C. tweediana, C. salicifolia, C. pallida, and A. emaraginata were nondormant, some seeds of each species had PD. Also, some seeds of M. detinens were nondormant and others had PY. In general, dormancy-break in seeds with PD supports our second hypothesis that dormancy-break is promoted by the environmental conditions in the habitat after the seeds are dispersed. Further, warm-wet conditions are optimal for dormancy-break of the relatively small fraction of the seed population of C. tweediana, C. salicifolia, and A. emarginata that has PD but not seeds of C. pallida. However, these conditions may not occur until the subsequent rainy period, in which case we suggest that seeds remain in the soil seed bank for several months before dormancy is broken. Similarly, in a tropical seasonal rain forest in Panama, the seeds dispersed at the end of the rainy season or during the dry season will not germinate until the following wet season (Garwood 1983; Sautu et al. 2006). In a seasonal grassland in Brazil, Mimosa foliolosa seeds are dispersed in the middle of the dry season and after storage in room dry conditions the germination increased probably due to seed coat softening (Silveira et al. 2014).
About 33% of the seeds of Celtis pallida had PD, but it was not broken by treatment with GA3, manual scarification, cold stratification, or warm stratification, suggesting that the seeds might have intermediate or deep PD. If the seeds have deep PD, the excised embryo will not grow or produce only a dwarf plant (Nikolaeva 1969), and many months of warm stratification may be required for dormancy break (Baskin et al. 2005). If the seeds have intermediate PD, warm followed by cold stratification may be effective for dormancy-break (Nikolaeva 1969). It is interesting to note that germination of C. pallida seeds was increased by exposing seeds to 3 days of wet heat at 30 °C followed by 2 weeks of cold stratification (Fulbright et al. 1986). Clearly more research is needed on the portion of the seed crop of C. pallida that has PD.
A relatively short period of warm stratification broke the dormancy in C. tweedieana seeds; thus, we concluded that they have nondeep PD (sensu Nikolaeva 1969; Baskin and Baskin 2014). The best dormancy- breaking treatment was 6 weeks of warm stratification, which reflects the conditions in the field when seeds of this species are dispersed at the beginning of the warm wet season. Cold and dry conditions were suitable for seed storage of this species. However, cold stratification did not increase germination and increased the percentage of dead seeds. Therefore, probably after dispersal, the seeds that did not germinate during the favorable season enter the soil seed bank where the cold dry conditions during the non-favorable season for germination maintain dormancy. During the subsequent favorable period, the temperature increases, and the rainy period produces a warm stratification that breaks dormancy allowing for germination. Dry storage at room temperatures did not increase germination of C. tweedieana seeds and lead to an increase in percentage of dead seeds; thus, storing seeds at room temperature is not recommended.
About 30% of seeds of C. salicifolia and 23% of seeds of Atamisquea emarginata were dormant and possibly possess deep PD because neither GA3, cold scarification, nor manually scarification promoted germination, and they did not afteripen (Nikolaeva 1969; Baskin and Baskin 2014). To be sure that seeds of these species have deep PD we should perform the treatments mentioned previously for C. pallida seeds. Warm stratification increased the germination of A. emarginata and C. salicifolia but to only 7.0% in both species. Furthermore, after 3-weeks of warm stratification the seeds of C. salicifolia died. Thus, more research is needed to determine the dormancy-breaking requirements for seeds of these two species, including extended periods of cold or warm stratification. For example, the germination of Capparis ovata was enhanced up to 46.6% after 60 days of cold stratification (Olmez et al. 2004). In contrast, the tropical montane species Leptecophylla tameiameiae required extended periods (162 days) of warm stratification to germinate (Baskin et al. 2005). The results of the afterripening experiment (i.e. dormancy-break during dry storage) demonstrate that we can store the seeds of A. emarginata and C. salicifolia dry at room temperature for 12-weeks without loss of viability.
Manual scarification was the most effective treatment to break PY of Mimosa detinens seeds than acid scarification; however, acid scarification could be useful to treat large numbers of seeds for sapling production in restoration programs. All soaking times tested were equally effective, thus we recommend 5 min of soaking in acid to save operation efforts.
Many fresh seeds of all species can germinate to high percentages. Further, seeds germinated better in light or equally well in light and dark (except for C. coccinea), and there was no effect of incubation at alternating vs. constant temperatures on germination in seven species. Germination of 17 woody species from Dry Chaco Forest in Argentina (including Celtis ehrenbergiana) was indifferent to light conditions and their germination was only promoted by the temperatures that occur during the warm-wet period (favorable season) (Funes et al. 2009). Thus, based on our results and the available information for Chaco species, we suggest that planting seeds on-site as soon as they mature via direct sowing in open spaces in the overgrazed places (with the exception of C. coccinea whose seeds will need to be covered by soil or litter) potentially is a good strategy for reintroduction of keystone-nurse-woody species to the open spaces in Dry Chaco Forest. However, the ability of seeds to germinate in the field has not been tested as there might be other factors, such as predation, that decreased their germination success, and thus seeds with only a short period of viability may need to be sown in a nursery. Based on light and temperature requirements, a similar recommendation was made for Guaiacum sanctum seeds. Since temperature mainly regulates seed germination of this species, it potentially can be introduced to open and partially-open sites to restore degraded areas of dry forests in Nicaragua (González-Rivas et al. 2009).
Seeds of some trees species from tropical dry forests lose viability during dry cold storage and even at room temperatures (Khurana and Singh 2001), so it is important to determinate proper seed storage procedures for each species. Although we did not study seed storage specifically, we could use our data to suggest some guidelines. Dry storage of seeds of C. coccinea, C. tweedieana, A. praecox, C. pallida, and M. detinens at 4 °C from time of dispersal until the following favorable season for germination is useful to retain seed viability. However, fresh seeds of C. retusa, A. speciosa, C. salicifolia, or A. emarginata should be sown immediately after collection. More research is needed to determine long term storage of these species. For example, seeds of Mimosa foliolosa retained viability during 3 years of dry storage at room temperature (Silveira et al. 2014).
Conclusions
Seeds of the nine keystone-nurse-woody species germinated to a high percentage and can be used for restoration purposes if they are fresh. We suggest planting seeds of the nine species in a nursery (or possibly on-site) as soon as they mature. Most fresh viable seeds are nondormant, but viability decreases during dry-cold storage restricting the use of some species. C. coccinea, A. praecox, M. detinens, and C. tweedieana are the most suitable keystone species for the restoration of overgrazed areas in the Dry Chaco Forest because their seeds germinate to a high percentage and retain viability during dry storage at low temperatures. Seeds of M. detinens and C. tweedieana require mechanical scarification and 6-weeks of warm stratification, respectively, for dormancy-breaking. Cynophalla retusa and A. speciosa have nondormant seeds that remain viable for only 1 month. Celtis pallida remained viable during cold-dry storage, but the degree of physiological dormancy increased. Atamisquea emarginata and C. salicifolia are not recommended as keystone species unless the seeds can be sown immediately after harvesting; most fresh viable seeds are nondormant, but viability decreases during dry-cold storage. Finally, the variation in dormancy and germination found in the Chaco species included in our study emphasizes the importance of conducting detailed studies on dormancy-breaking and germination requirements of species that are deemed to be of high value in forest restoration efforts.
Data availability
Permission for seed collection was provided by the National Park Administration of Argentina (project NEA 421). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abraham de Noir F, Bravo S (2014) Frutos de Leñosas Nativas del Chaco. UNSE Press, Santiago del Estero
Abril A, Bucher EH (1999) The effects of overgrazing on soil microbial community and fertility in the Chaco dry savannas of Argentina. Appl Soil Ecol 12:1591–2167. https://doi.org/10.1016/S0929-1393(98)00162-0
Aronson J, Floret C, Le Floch E, Ovalle C, Pontanier R (1993) Restoration and rehabilitation of degraded ecosystems in arid and semi-arid lands. I. A view from the South. Restor Ecol 1(1):81–17
Barchuk AH, Iglesias MDR, Boetto MN (2008) Spatial association of Aspidosperma quebracho-blanco juveniles with shrubs and conspecific adults in the Arid Chaco, Argentina. Austral Ecol 33:7751–7783. https://doi.org/10.1111/j.1442-9993.2008.01846.x
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16. https://doi.org/10.1079/ssr2003150
Baskin CC, Baskin JM (2014) Seeds. Ecology, biogeography, and evolution of dormancy and evolution, 2nd edn. Academic Press, San Diego
Baskin CC, Baskin JM (2022) Invited review: mimicking the natural thermal environments experienced by seeds to break physiological dormancy to enhance seed testing and seedling production. Seed Sci Technol 50(1–2):1–2. https://doi.org/10.15258/sst.2022.50.1.s.02
Baskin CC, Baskin JM, Yoshinaga A, Thompson K (2005) Germination of drupelets in multi-seeded drupes of the shrub Leptecophylla tameiameiae (Ericaceae) from Hawaii: a case for deep physiological dormancy broken by high temperatures. Seed Sci Res 15(4):349–356. https://doi.org/10.1079/SSR2005223
Beniwal BS, Singh NB (1989) Observations on flowering, fruiting and germination behaviours of some useful forest plants of Arunachal Pradesh. Indian for 115:216–227
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Bianchi AR, Yáñez CE (1992) Las precipitaciones del Noroeste Argentino. Instituto Nacional de Tecnología Agropecuaria, Salta
Boletta PE, Ravelo AC, Planchuelo AM, Grilli M (2006) Assessing deforestation in the Argentine Chaco. For Ecol Manag 228:108–114. https://doi.org/10.1016/j.foreco.2006.02.045
Breshears DD, Nyhan JW, Heil CE, Wilcox BP (1998) Effects of woody plants on microclimate in a semiarid woodland: soil temperature and evaporation in canopy and intercanopy patches. Int J Plant Sci 159:1010–1017. https://doi.org/10.1086/314083
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34. https://doi.org/10.1111/j.1365-2745.2007.01295.x
Bucher EH (1982) Chaco and Caatinga-South American arid savannas, woodlands and thickets. Ecol Trop Savannas 8:48–79
Budelsky RA, Galatowitsch SM (1999) Effects of moisture, temperature, and time on seed germination of five wetland Carices: implications for restoration. Restor Ecol 7(1):86–97. https://doi.org/10.1046/j.1526-100X.1999.07110.x
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Castro J, Zamora R, Hódar JA, Gómez JM, Gómez-Aparicio L (2004) Benefits of using shrubs as nurse plants for reforestation in Mediterranean mountains: a 4-year study. Restor Ecol 12:352–358. https://doi.org/10.1111/j.1061-2971.2004.0316.x
Cervantes M, Ceccon E, Bonfil C (2014) Germination of stored seeds of four tree species from the tropical dry forest of Morelos. Mexico Bot Sci 92(2):281–287
Czeglédi L, Radácsi A (2005) Overutilization of pastures by livestock. Grassl Stud 3:29–36
De Vitis M, Hay FR, Dickie JB, Trivedi C, Choi J, Fiegener R (2020) Seed storage: maintaining seed viability and vigor. Restor Ecol 28:S249–S255. https://doi.org/10.1111/rec.13174
Duncan C, Schultz N, Lewandrowski W, Good MK, Cook S (2019) Lower dormancy with rapid germination is an important strategy for seeds in an arid zone with unpredictable rainfall. PLoS ONE 14(9):e0218421. https://doi.org/10.1371/journal.pone.0218421
Elliott S, Kuarak C, Navakitbumrung P, Zangkum S, Anusarnsunthorn V, Blakesley D (2002) Propagating framework trees to restore seasonally dry tropical forest in northern Thailand. New for 23:63–70. https://doi.org/10.1023/A:1015641119271
Elliott S, Navakitbumrung P, Kuarak C, Zangkuma S, Anusarnsunthorna V, Blakesley D (2003) Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. For Ecol Manag 184:177–191. https://doi.org/10.1016/S0378-1127(03)00211-1
Escobar DFE, Silveira FAO, Morellato LPC (2018) Timing of seed dispersal and seed dormancy in Brazilian savanna: two solutions to face seasonality. Ann Bot 121:1197–1209. https://doi.org/10.1093/aob/mcy006
Flores J, Jurado E (2003) Are nurse-protege interactions more common among plants from arid environments? J Veg Sci 14:911–916. https://doi.org/10.1111/j.1654-1103.2003.tb02225.x
Franceschini MC, Tressens SG (2004) Morphology of fruits, seeds and embryos of Argentinian Capparis L. (Capparaceae). Bot J Linn 145(2):209–218. https://doi.org/10.1111/j.1095-8339.2003.00279.x
Fulbright TE, Flenniken KS, Waggerman GL (1986) Enhancing germination of spiny hackberry seeds. J Range Manag 39:552–554. https://doi.org/10.2307/3898769
Funes G, Díaz S, Venier P (2009) La temperatura como principal determinante de la germinación en especies del Chaco seco de Argentina. Ecol Austral 19:129–138
Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC (2012) The autumn effect: timing of physical dormancy break in seeds of two winter annual species of Geraniaceae by a stepwise process. Ann Bot 110:637–651. https://doi.org/10.1093/aob/mcs122
Garwood NC (1983) Seed germination in a seasonal tropical forest in Panama: a community study. Ecol Monogr 53:159–181. https://doi.org/10.2307/1942493
Gasparri NI, Grau HR (2009) Deforestation and fragmentation of Chaco dry forest in NW Argentina (1972–2007). For Ecol Manag 258:913–921. https://doi.org/10.1016/j.foreco.2009.02.024
Gómez-Aparicio L (2009) The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J Ecol 97:1202–1214. https://doi.org/10.1111/j.1365-2745.2009.01573.x
Gómez-Aparicio L, Zamora R, Gómez JM, Hódar JA, Castro J, Baraza E (2004) Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol Appl 14:1128–1138. https://doi.org/10.1890/03-5084
González-Rivas B, Tigabu M, Castro-Marín G, Odén PC (2009) Seed germination and seedling establishment of Neotropical dry forest species in response to temperature and light conditions. J for Res 20:99–104. https://doi.org/10.1007/s11676-009-0018-y
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavinada A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A, Egorov A, Chini L, Justice CO, Townshend JRG (2013) High-resolution global maps of 21st-century forest cover change. Science 342:850–854. https://doi.org/10.1126/science.1244693
Holl KD (2002) Effect of shrubs on tree seedling establishment in an abandoned tropical pasture. J Ecol 90:179–187. https://doi.org/10.1046/j.0022-0477.2001.00637.x
Instituto de Botánica Darwinion (2022) www.darwin.edu.ar
INTA, Instituto Nacional de Tecnología Agropecuaria (2020) www.inta.gov.ar
Jurado E, Flores J (2005) Is seed dormancy under environmental control or bound to plant traits? J Veg Sci 16:559–564. https://doi.org/10.1111/j.1654-1103.2005.tb02396.x
Khurana E, Singh JS (2001) Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environ Conserv 28:39–52. https://doi.org/10.1017/S0376892901000042
Kuemmerle T, Altrichter M, Baldi G, Cabido M, Camino M, Cuellar E, Cuellar RL, Decarre J, Díaz D, Gasparri I, Gavier-Pizarro G, Ginzburg R, Giordano AJ, Grau HR, Jobbágy E, Leynaud G, Macchi L, Mastrangelo M, Matteucci SD, Noss A, Paruelo J, Piquer-Rodríguez M, Romero-Muñoz A, Semper-Pascual A, Thompson J, Torrella S, Torres R, Volante JN, Yanosky A, Zak M (2017) Forest conservation: remember Gran Chaco. Science 355(6324):465–466. https://doi.org/10.1126/science.aal5010
Lieberman D, Li M (1992) Seedling recruitment patterns in a tropical dry forest in Ghana. J Veg Sci 3:375–382. https://doi.org/10.2307/3235763
Macchi L, Grau HR (2012) Piospheres in the dry Chaco. Contrasting effects of livestock puestos on forest vegetation and bird communities. J Arid Environ 87:176–187. https://doi.org/10.1016/j.jaridenv.2012.06.003
Martínez-Carretero E, Dalmasso A (2015) Revegetación de ambientes degradados. Selección de especies. In: Martínez-Carretero E, Dalmasso A (eds) Restauración ecológica en la Diagonal Árida de la Argentina 2, 1st edn. Mendoza, Argentina, pp 21–30
Mazzini F, Relva MA, Malizia LR (2018) Impacts of domestic cattle on forest and woody ecosystems in southern South America. Plant Ecol 219:913–925. https://doi.org/10.1007/s11258-018-0846-y
McDonald T, Gann GD, Jonson J, Dixon KW (2016) International standards for the practice of ecological restoration—including principles and key concepts. Society for Ecological Restoration, Washington
Meli P, Martínez-Ramos M, Rey-Benayas JM, Carabias J (2014) Combining ecological, social and technical criteria to select species for forest restoration. Appl Veg Sci 17:744–753. https://doi.org/10.1111/avsc.12096
Morello JH, Rodriguez AF (2009) El Chaco sin bosques: la pampa o el desierto del futuro. Orientación Gráfica Press, Buenos Aires
Navarro-Cano JA, Goberna M, Verdú M (2019) La facilitación entre plantas como herramienta de restauración de diversidad y funciones ecosistémicas. Ecosistemas 28:20–31. https://doi.org/10.7818/ECOS.1747
Nikolaeva MG (1969) Fizilogiya glubokogo pokoya semyan (Physiology of deep dormancy in seeds). Leningrad, Nauka. Translated from Russian to English by Z. Shapiro, National Science Foundation, Washington
Olmez Z, Ucler AO, Yahyaoglu Z (2004) Effects of stratification and chemical treatments on germination of caper (Capparis ovata Desf.) seeds. Agric Med 134(2):101–106
Oscar-Palacio M, Roger E (2016) Árboles Autóctonos de Santiago del Estero: guía para su reconocimiento en el Jardín Botánico Ing. Lucas D. Roic. UNSE Press, Santiago del Estero
Padilla FM, Pugnaire FI (2006) The role of nurse plants in the restoration of degraded environments. Front Ecol Environ 4:196–202. https://doi.org/10.1890/1540-9295(2006)004[0196:TRONPI]2.0.CO;2
Padilla FM, Ortega R, Sánchez J, Pugnaire FI (2009) Rethinking species selection for restoration of arid shrublands. Basic Appl Ecol 10:640–647. https://doi.org/10.1016/j.baae.2009.03.003
Padilla-Ruiz FM, Pugnaire De Idaola FI (2004) El uso de especies arbustivas para la restauración de la cubiertavegetal en ambientes semiáridos. Cuad La Soc Española Ciencias for 107:103–107
Páez SA, Marco DE (2000) Seedling habitat structure in dry Chaco forest (Argentina). J Arid Environ 46:57–68. https://doi.org/10.1006/jare.2000.0648
Peña-Chocarro MC, De Egea JJ, Vera M, Maturo H, Knapp S (2006) Guía de Árboles y Arbustos del Chaco Húmedo. The History Museum Guyra-Paraguay. Fundación Moisés Bertoni y Fundación Hábitat y Desarrollo, Asunción
Pérez DR, Farinaccio FM, Aronson J (2019) Towards a dryland framework species approach. Research in progress in the Monte Austral of Argentina. J Arid Environ 161:1–10. https://doi.org/10.1016/j.jaridenv.2018.09.001
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramos DM, Diniz P, Ooi MKJ, Borghetti F, Valls JFM (2017) Avoiding the dry season: dispersal time and syndrome mediate seed dormancy in grasses in Neotropical savanna and wet grasslands. J Veg Sci 28:798–807. https://doi.org/10.1111/jvs.12531
Ray GJ, Brown BJ (1995) Restoring caribbean dry forests: evaluation of tree propagation techniques. Restor Ecol 3:86–94. https://doi.org/10.1111/j.1526-100X.1995.tb00081.x
Rey Benayas JM, Bullock JM, Newton AC (2008) Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front Ecol Environ 6:329–336. https://doi.org/10.1890/070057
Salazar A, Goldstein G, Franco AC, Miralles-Wilhelm F (2011) Timing of seed dispersal and dormancy, rather than persistent soil seed-banks, control seedling recruitment of woody plants in Neotropical savannas. Seed Sci Res 21:103–116. https://doi.org/10.1017/S0960258510000413
Sautu A, Baskin JM, Baskin CC, Condit R (2006) Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. For Ecol Manag 234:245–263. https://doi.org/10.1016/j.foreco.2006.07.006
Silveira FA, Negreiros D, Ranieri BD, Silva CA, Araújo LM, Fernandes W (2014) Effect of seed storage on germination, seedling growth and survival of Mimosa foliolosa (Fabaceae): implications for seed banks and restoration ecology. Trop Ecol 55(3):385–392
Tálamo A, Barchuk A, Cardozo S, Trucco C, Marás G, Trigo C (2015a) Direct versus indirect facilitation (herbivore mediated) among woody plants in a semiarid Chaco forest: a spatial association approach. Austral Ecol 40:573–580. https://doi.org/10.1111/aec.12224
Tálamo A, Barchuk AH, Garibaldi LA, Trucco CE, Cardozo S, Mohr F (2015b) Disentangling the effects of shrubs and herbivores on tree regeneration in a dry Chaco forest (Argentina). Oecologia 178:847–854. https://doi.org/10.1007/s00442-015-3269-7
van Schaik CP, Terborgh JW, Wright SJ (1993) The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annu Rev Ecol Syst 24:353–377. https://doi.org/10.1146/annurev.es.24.110193.002033
Waiboonya P, Elliott S (2020) Sowing time and direct seeding success of native tree species for restoring tropical forest ecosystems in northern Thailand. New for 51(1):81–99. https://doi.org/10.1007/s11056-019-09720-1
Walter H, Burnett JH (1971) Ecology of tropical and subtropical vegetation. Oliver and Boyd, Edinburgh
Zak MR, Cabido M, Hodgson JG (2004) Do subtropical seasonal forests in the Gran Chaco, Argentina, have a future? Biol Conserv 120:589–598. https://doi.org/10.1016/j.biocon.2004.03.034
Zamith LR, Scarano FR (2004) Producao de mudas de especies da Restingas do Municipio do Rio de Janeiro, RJ, Brasil. Acta Bot Brasil 18:161–176
Acknowledgements
We thank the authorities from Copo National Park and the local community for permission and assistance with field work. We also thank our field and lab assistants, especially Lucia Lindow, Manuela Urtasun, and Diego López-Spahr.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by National Council of Science and Technology (CONICET) from Argentina (PhD fellowship) and the Fulbright foundation (Fulbright Scholarship Program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Gálvez, F., Baskin, C.C., Croce, J. et al. Propagation of keystone-woody species as a first step in restoration of an overgrazed seasonal dry forest. New Forests 55, 363–382 (2024). https://doi.org/10.1007/s11056-023-09977-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-023-09977-7