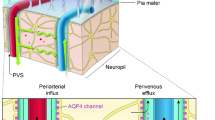
Selective and progressive neuron death is a characteristic feature of the neurodegenerative process and leads to corresponding neuron dysfunctions. Neurodegenerative diseases constitute a heterogeneous group of nosologies with different clinical presentations but similar molecular mechanisms of pathogenesis. They are based on processes of abnormal protein aggregation and the formation of fibrillar insoluble structures and their deposition as histopathological inclusions in nervous system tissues. Disruption of homeostatic functions regulating neuron ion and energy metabolism, protein and nucleotide biosynthesis and degradation, chronic hypoxia, and penetration of toxic and inflammatory substances into the brain from the bloodstream not only cause age-related metabolic changes and disturbances in the sleep–waking cycle, but also contribute to the development of neurodegenerative processes. Animal studies have identified pathways of clearance in which solutes and specific tracers are cleared by a perivascular pathway into meningeal lymphatic vessels. The glymphatic network facilitates the clearance of metabolites, including beta-amyloid and tau protein, from the extracellular space of the brain. The glymphatic system is more efficient during natural sleep; fluid dynamics through this pathway display daily fluctuations and are under circadian control. The review systematizes the key aspects and scientific data from recent studies on the role of the glymphatic pathway and astroglial aquaporin type 4 as its main determinant in maintaining homeostatic fluid circulation in the brain in normal and pathological conditions, particularly in relation to the regulatory role of the sleep–waking cycle and during the development of neurodegenerative processes.
Similar content being viewed by others
References
Iliff, J. J., Wang, M., Liao, Y., et al., “A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta,” Sci. Transl. Med., 4, No. 147, 147ra11 (2012), https://doi.org/10.1126/scitranslmed.3003748.
Kress, B., Iliff, J., Xia, M., et al., “Impairment of paravascular clearance pathways in the aging brain,” Ann. Neurol., 76, No. 6, 845–861 (2014), https://doi.org/10.1002/ana.24271.
Rasmussen, M. K., Mestre, H., and Nedergaard, M., “The glymphatic pathway in neurological disorders,” Lancet Neurol., 17, No. 11, 1016–1024 (2018), https://doi.org/10.1016/S1474-4422(18)30318-1.
Tithof, J., Boster, K. A. S., Bork, P. A. R., et al., “Network model of glymphatic flow under different experimentally-motivated parametric scenarios,” iScience, 25, No. 5, 104258 (2022), https://doi.org/10.1016/j.isci.2022.104258.
Vasciaveo, V., Iadarola, A., Casile, A., et al., “Sleep fragmentation affects glymphatic system through the different expression of AQP4 in wild type and 5xFAD mouse models,” Acta Neuropathol. Comm., 11, 16 (2023), https://doi.org/10.1186/s40478-022-01498-2.
Goedert, M., “Alzheimer’s and Parkinson’s diseases; The prion concept in relation to assembled Ab, tau, and a-synuclein,” Science, 349, 1255555 (2015), https://doi.org/10.1126/science.1255555.
Diack, A. B., Alibhai, J. D., Barron, R., et al., “Insights into Mechanisms of Chronic Neurodegeneration,” Int. J. Mol. Sci., 17, No. 1, 82 (2016), https://doi.org/10.3390/ijms17010082.
Tarutani, A., Adachi, T., Akatsu, H., et al., “Ultrastructural and biochemical classification of pathogenic tau, α-synuclein and TDP-43,” Acta Neuropathol., 143, No. 6, 613–640 (2022), https://doi.org/10.1007/s00401-022-02426-3.
Huang, M. and Chen, S., “DJ-1 in neurodegenerative diseases: Pathogenesis and clinical application,” Prog. Neurobiol., 204, 102114 (2021), https://doi.org/10.1016/j.Pneurobio.2021.102114.
Chen H-L, Chen P-C, Lu C-H, et al., “Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson’s disease,” Oxid. Med. Cell. Longev., 2021, 4034509 (2021), https://doi.org/10.1155/2021/4034509.
Hernaiz, A., Toivonen, J. M., Bolea, R., et al., “Epigenetic changes in prion and prion-like neurodegenerative diseases: Recent advances, potential as biomarkers and future perspectives,” Int. J. Mol. Sci., 23, No. 20, 12609 (2022), https://doi.org/10.3390/ijms232012609.
Fang Y-C, Hsieh Y-C, Hu C-J, et al., “Endothelial dysfunction in neurodegenerative diseases,” Int. J. Mol. Sci., 24, No. 3, 2909 (2023), https://doi.org/10.3390/ijms24032909.
Li, K. and Wang, Z., “lncRNA NEAT1: Key player in neurodegenerative diseases,” Ageing Res. Rev., 86, 101878 (2023), https://doi.org/10.1016/j.arr.2023.101878.
Carrera-Gonzalez, M. D. P., Canton-Habas, V., and Rich-Ruiz, M., “Aging, depression and dementia: The inflammatory process,” Adv. Clin. Exp. Med., 31, 469–473 (2022), https://doi.org/10.17219/acem/149897.
Mehta, N. H., Suss, R. A., Dyke, J. P., et al., “Quantifying cerebrospinal fluid dynamics: a review of human neuroimaging contributions to CSF physiology and neurodegenerative disease,” Neurobiol. Dis., 170, 105776 (2022), https://doi.org/10.1016/j.nbd.2022.105776.
Xie, L., Kang, H., Xu, Q., et al., “Sleep drives metabolite clearance from the adult brain,” Science, 342, No. 6156, 373–377 (2013), https://doi.org/10.1126/science.1241224.
Tang, J., Zhang, M., Liu, N., et al., “The Association Between Glymphatic System Dysfunction and Cognitive Impairment in Cerebral Small Vessel Disease,” Front. Aging Neurosci., 14, 916633 (2022), https://doi.org/10.3389/fnagi.2022.916633.
Carotenuto, A., Cacciaguerra, L., Pagani, E., et al., “Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability,” Brain, 145, No. 8, 2785–2795 (2022), https://doi.org/10.1093/brain/awab454.
Albargothy, N., Johnston, D., MacGregor-Sharp, M., et al., “Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways,” Acta Neuropathol., 136, No. 1, 139–152 (2018), https://doi.org/10.1007/s00401-018-1862-7.
Carare, R., Aldea, R., Agarwal, N., et al., “Clearance of interstitial fluid and CSF group – part of vascular professional interest area,” Alzheimers Dement., 12, No. 1, e12053 (2020), https://doi.org/10.1002/dad2.12053.
Gouveia-Freitas, K. and Bastos-Leite, A. J., “Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology,” Neuroradiology, 63, No. 10, 1581–1597 (2021), https://doi.org/10.1007/s00234-021-02718-7.
Wu, C.-H., Lirng, J.-F., Ling, Y.-H., et al., “Noninvasive characterization of human Glymphatics and meningeal lymphatics in an in vivo model of blood–brain barrier leakage,” Ann. Neurol., 89, No. 1, 111–124 (2021), https://doi.org/10.1002/ana.25928.
Iliff, J. J., Lee, H., Yu, M., et al., “Brain-wide pathway for waste clearance captured by contrast-enhanced, MRI,” J. Clin. Invest., 123, No. 3, 1299–1309 (2013), https://doi.org/10.1172/JCI67677.
Ringstad, G., Valnes, L. M., et al., “Brain-wide glymphatic enhancement and clearance in humans assessed with, MRI,” JCI Insight, 3, No. 13, 121537 (2018), https://doi.org/10.1172/jci.insight.121537.
Eide, P. R., Vatnehol, S., Emblem, K., et al., “Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes,” Sci. Rep., 8, No. 1, 7194 (2018), https://doi.org/10.1038/s41598-018-25666-4.
Nedergaard, M. and Goldman, S. A., “Glymphatic failure as a final common pathway to dementia,” Science, 370, No. 6512, 50–56 (2020), https://doi.org/10.1126/science.abb8739.
Dudchenko, N. G., Chimagomedova, A. Sh., Vasenina, E. E., and Levin, O. S., “The glymphatic system,” Zh. Nevrol. Psikhiatr., 122, No. 7, 20–26 (2022), https://doi.org/10.17116/jnevro202212207120.
Ishida, K., Yamada, K., Nishiyama, R., et al., “Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration,” J. Exp. Med., 219, No. 3, 20211275 (2022), https://doi.org/10.1084/jem.20211275.
Harrison, I., Ismail, O., Machhada, A., et al., “Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model,” Brain, 143, No. 8, 2576–2593 (2020), https://doi.org/10.1093/brain/awaa179.
Zhang, J., Zhao, H., Xue, Y., et al., “Impaired glymphatic transport kinetics following induced acute ischemic brain edema in a mouse pMCAO model,” Front. Neurol., 13, 860255 (2022), https://doi.org/10.3389/fneur.2022.860255.
Mestre, H., Hablitz, L., Xavier, A., et al., “Aquaporin-4-dependent glymphatic solute transport in the rodent brain,” eLife, 7, e40070 (2018), https://doi.org/10.7554/eLife.40070.
Simon, M., Wang, M. X., Ismail, O., et al., “Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid beta plaque formation in mice,” Alzheimers Res. Ther., 14, No. 1, 59 (2022), https://doi.org/10.1186/s13195-022-00999-5.
Marin-Moreno, A., Canoyra, S., Fernandez-Borges, N., et al., “Transgenic mouse models for the study of neurodegenerative diseases,” Front. Biosci., 28, No. 1, 21 (2023), https://doi.org/10.31083/j.fbl2801021.
Hablitz, L. and Nedergaard, M., “The glymphatic system: a novel component of fundamental neurobiology,” J. Neurosci., 41, No. 37, 7698–7711 (2021), https://doi.org/10.1523/JNEUROSCI.0619-21.2021.
Salman, M. M., Kitchen, P., Iliff, J. J., et al., “Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis,” Nat. Rev. Neurosci., 22, 650–651 (2021), https://doi.org/10.1038/s41583-021-00514-z.
Zhang, R., Liu, Y., and Chen, Y., et al., “Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption,” CNS Neurosci. Ther., 26, No. 2, 228–239 (2020), https://doi.org/10.1111/cns.13194.
Wang, M. X., Ray, L., Tanaka, K., et al., “Varying perivascular astroglial endfoot dimensions along the vascular tree maintain perivascular-interstitial flux through the cortical mantle,” Glia, 69, No. 3, 715–728 (2021), https://doi.org/10.1002/glia.23923.
Hladky, S. B. and Barrand, M. A., “The glymphatic hypothesis: the theory and the evidence,” Fluids Barriers CNS, 19, No. 1, 9 (2022), https://doi.org/10.1186/s12987-021-00282-z.
Xu, Z., Xiao, N., Chen, Y., et al., “Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits,” Mol. Neurodegener., 10, No. 1, 1–16 (2015), https://doi.org/10.1186/s13024-015-0056-1.
Kondrat’ev, A. N. and Tsentsiper, L. M., “The glymphatic system of the brain: structure and practical significance,” Anesteziol. Reanimatol., 6, 72–80 (2019), https://doi.org/10.17116/anaesthesiology201906172.
Mogensen, F. L. H., Delle, C., and Nedergaard, M., “The glymphatic system during inflammation,” Int. J. Mol. Sci., 22, No. 14, 7491 (2021), https://doi.org/10.3390/ijms22147491.
Keil, S. A., Braun, M., O’Boyle, R., et al., “Dynamic infrared imaging of cerebrospinal fluid tracer influx into the brain,” Neurophotonics, 9, No. 3, 031915 (2022), https://doi.org/10.1117/1.NPh.9.3.031915.
Zeppenfeld, D. M., Simon, M., Haswell, J. D., et al., “Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains,” JAMA Neurol., 74, No. 1, 91–99 (2017), https://doi.org/10.1001/jamaneurol.2016.4370.
Arighi, A., Arcaro, M., Fumagalli, G. G., et al., “Aquaporin-4 cerebrospinal fluid levels are higher in neurodegenerative dementia: looking at glymphatic system dysregulation,” Alzheimers Res. Ther., 14, No. 1, 135 (2022), https://doi.org/10.1186/s13195-022-01077-6.
Haveke, R., Park, A. J., Tudor, J. C., et al., “Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1,” eLife, 5, e13424 (2016), https://doi.org/10.7554/eLife.13424.
Fultz, N. E., Bonmassar, G., Setsompop, K., et al., “Coupled electrophysiological, hemodynamic and cerebrospinal fluid oscillations in human sleep,” Science, 366, 628–631 (2019), https://doi.org/10.1126/science.aax5440.
Li, H., Yu, F., Sun, X., et al., “Dihydromyricetin ameliorates memory impairment induced by acute sleep deprivation,” Eur. J. Pharmacol., 853, 220–228 (2019), https://doi.org/10.1016/j.ejphar.2019.03.014.
Jack CR Jr, Wiste, H. J., Weigand, S. D., et al., “Age, sex, and APOE ε4 effects on memory, brain structure, and beta-amyloid across the adult life span,” JAMA Neurol., 72, No. 5, 511–519 (2015), https://doi.org/10.1001/jamaneurol.2014.4821.
Bah, T. M., Goodman, J., and Iliff, J. J., “Sleep as a therapeutic target in the aging brain,” Neurotherapeutics, 16, No. 3, 554–568 (2019), https://doi.org/10.1007/s13311-019-00769-6.
Ju, Y. S., Ooms, S. J., Sutphen, C., et al., “Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels,” Brain, 140, No. 8, 2104–2111 (2017), https://doi.org/10.1093/brain/awx148.
Holth, J. K., Fritschi, S. K., Wang, C., et al., “The sleep–wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans,” Science, 363, 880–884 (2019), https://doi.org/10.1126/science.aav2546.
Hablitz, L., Pla, V., and Giannetto, M., et al., “Circadian control of brain glymphatic and lymphatic fluid flow,” Nat. Commun., 11, No. 1, 4411 (2020), https://doi.org/10.1038/s41467-020-18115-2.
Achariyar, T. M., Li, B., Peng, W., et al., “Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation,” Mol. Neurodegener., 11, No. 1, 74 (2016), https://doi.org/10.1186/s13024-016-0138-8.
Rainey-Smith, S. R., Mazzucchelli, G. N., Villemagne, V. L., et al., “Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden,” Transl. Psychiatry, 8, No. 1, 47 (2018), https://doi.org/10.1038/s41398-018-0094-x.
Burfeind, K. G., Murchison, C. F., Westaway, S. K., et al., “The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease,” Alzheimers Dement., 3, No. 3, 348–359 (2017), https://doi.org/10.1016/j.trci.2017.05.001.
Bah, T. M., Siler, D. A., Ibrahim, A. H., et al., “Fluid dynamics in aging-related dementias,” Neurobiol. Dis., 177, 105986 (2023), https://doi.org/10.1016/j.nbd.2022.105986.
Zhou, Y., Cai, J., Zhang, W., et al., “Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human,” Ann. Neurol., 87, No. 3, 357–369 (2020), https://doi.org/10.1002/ana.25670.
Kamagata, K., Andica, C., Takabayashi, K., et al., “Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease,” Neurology, 99, No. 24, 2648–2660 (2022), https://doi.org/10.1212/WNL.0000000000201300.
Gordleeva, S., Kanakov, O., Ivanchenko, M., et al., “Modelling the role of sleep, glymphatic system, and microglia senescence in the propagation of inflammaging,” Semin. Immunopathol., 42, No. 5, 647–665 (2020), https://doi.org/10.1007/s00281-020-00816-x.
Soden, P. A., Henderson, A. R., and Lee, E., “A microfluidic model of AQP4 polarization dynamics and fluid transport in the healthy and inflamed human brain: the first step towards glymphatics-on-a-chip,” Adv. Biol., 6, No. 12, e2200027 (2022), https://doi.org/10.1002/adbi.202200027.
Spitz, S., Ko, E., Ertl, P., et al., “How organ-on-a-chip technology can assist in studying the role of the glymphatic system in neurodegenerative diseases,” Int. J. Mol. Sci., 24, No. 3, 2171 (2023), https://doi.org/10.3390/ijms24032171.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 123, No. 9, pp. 31–36, September, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirolapov, I.V., Zakharov, A.V., Smirnova, D.A. et al. The Role of the Glymphatic Clearance System in the Mechanisms of the Interactions of the Sleep–Waking Cycle and the Development of Neurodegenerative Processes. Neurosci Behav Physi 54, 199–204 (2024). https://doi.org/10.1007/s11055-024-01585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-024-01585-y