Abstract
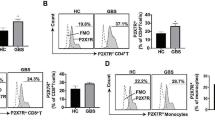
Wallerian degeneration following nerve injury not only suppresses CD4+ T-cell responses but also promotes pro-inflammatory immunological responses through TNF-α and IL-1β. Recent research suggests that thymoquinone might enhance nerve recovery by exerting anti-inflammatory effects on both the innate and adaptive immune systems. This study aims to evaluate the effect of thymoquinone on neuroinflammation in a sciatic nerve crush injury, as represented by TNF-α, IL-1β, and the CD4+:CD8+ ratio. In this study, 126 crush injury Wistar rats were divided into three main groups: placebo, thymoquinone 100 mg/kg, and thymoquinone 250 mg/kg administered daily. Rats were euthanized at six distinct time points: 12, 18, and 24 hours, as well as on day-5, day-6, and day-7. TNF-α and IL-1β levels were assessed using the Enzyme-Linked Immunosorbent Assay (ELISA). The CD4+:CD8+ ratio in peripheral blood was determined via flow cytometry. No significant TNF-α differences was found between treatment and placebo groups. However, on day 6, IL-1β was significantly lower in the TQ 250mg/kg group than in the placebo (p=0.008). A similar but non-significant trend existed on days 6 and 7. On day 5, both TQ groups showed a higher, statistically significant CD4+:CD8+ ratio compared to placebo (p=0.007), a trend that continued to day 7 but not statistically significant. Daily TQ administration did not consistently reduce TNF-α and IL-1ß levels. However, both doses elevated the CD4+:CD8+ ratio during the early stages of Wallerian degeneration, suggesting a potential benefit of TQ on nerve regeneration.


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request
References
Mietto BS, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J Neurosci 2015;35:16431–42. https://doi.org/10.1523/JNEUROSCI.2119-15.2015.
Wong K, Babetto E, Beirowski B. Axon degeneration: make the Schwann cell great again. Neural Regen Res 2017;12:518. https://doi.org/10.4103/1673-5374.205000.
Iwatsuki K, Arai T, Ota H, Kato S, Natsume T, Kurimoto S, et al. Targeting Anti-Inflammatory Treatment Can Ameliorate Injury-Induced Neuropathic Pain. PLoS One 2013;8. https://doi.org/10.1371/journal.pone.0057721.
Kato K, Liu H, Kikuchi SI, Myers RR, Shubayev VI. Immediate anti-tumor necrosis factor-α (etanercept) therapy enhances axonal regeneration after sciatic nerve crush. J Neurosci Res 2010;88:360–8. https://doi.org/10.1002/jnr.22202.
Wu R, Chen B, Jia X, Qiu Y, Liu M, Huang C, et al. Interleukin‐1 β influences functional regeneration following nerve injury in mice through nuclear factor‐ κ B signaling pathway. Immunology 2019;156:235–48. https://doi.org/10.1111/imm.13022.
Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger--damage control by the immune system. J Leukoc Biol 2012;92:539–51. https://doi.org/10.1189/JLB.0212072.
Pottoo FH, Ibrahim AM, Alammar A, Alsinan R, Aleid M, Alshehhi A, et al. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals (Basel) 2022;15:1–11. https://doi.org/10.3390/ph15040408.
Alkhattabi NA, Hussein SA, Tarbiah NI, Alzahri RY, Khalifa R. Thymoquinone Effect on Monocyte-Derived Macrophages, Cell-Surface Molecule Expression, and Phagocytosis. Nutrients 2022;14:1–13. https://doi.org/10.3390/nu14245240.
Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int Immunopharmacol 2015;28:295–304. https://doi.org/10.1016/j.intimp.2015.06.023.
Arumugam P, Subramanian R, Priyadharsini JV, Gopalswamy J. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin J Nat Med 2016;14:904–12. https://doi.org/10.1016/S1875-5364(17)30015-8.
Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep 2017;7:1–12. https://doi.org/10.1038/srep42995.
Farkhondeh T, Samarghandian S, Shahri AMP, Samini F. The Neuroprotective Effects of Thymoquinone: A Review. Dose-Response 2018;16:155932581876145. https://doi.org/10.1177/1559325818761455.
Ahmad A, Mishra RK, Vyawahare A, Kumar A, Rehman MU, Qamar W, et al. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm J 2019;27:1113–26. https://doi.org/10.1016/j.jsps.2019.09.008.
Goyal SN, Prajapati CP, Gore PR, Patil CR, Mahajan UB, Sharma C, et al. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front Pharmacol 2017;8:1–19. https://doi.org/10.3389/fphar.2017.00656.
Sereflican M, Yurttas V, Ozyalvacli G, Terzi EH, Turkoglu SA, Yildiz S, et al. The histopathological and electrophysiological effects of thymoquinone and methylprednisolone in a rabbit traumatic facial nerve paralysis model. Am J Otolaryngol - Head Neck Med Surg 2016;37:407–15. https://doi.org/10.1016/j.amjoto.2016.05.006.
Gülşen İ, Ak H, Kara M, Gökalp A, Akyol V, Koçak ÖF, et al. Timokinonun akut periferik sinir hasarı üzerine akut etkisi: Deneysel bir çalışma. Ulus Travma ve Acil Cerrahi Derg 2016;22:526–30. https://doi.org/10.5505/tjtes.2016.40204.
Cámara-Lemarroy CR, Guzmán-De La Garza FJ, Fernández-Garza NE. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 2010;17:314–24. https://doi.org/10.1159/000292020.
DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The Neuroimmunology of Degeneration and Regeneration in the Peripheral Nervous System. vol. 302. 2015. https://doi.org/10.1016/j.neuroscience.2014.09.027.The.
Rotshenker S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J Neuroinflammation 2011;8:109. https://doi.org/10.1186/1742-2094-8-109.
Chen L, Li B, Chen B, Shao Y, Luo Q, Shi X, et al. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Sci Rep 2016;6:1–11. https://doi.org/10.1038/srep31656.
Chen Y-M, Shen R-W, Zhang B, Zhang W-N. Regional tissue immune responses after sciatic nerve injury in rats. Int J Clin Exp Med 2015;8:13408–12.
Yuan W, Feng X. Immune cell distribution and immunoglobulin levels change following sciatic nerve injury in a rat model. Iran J Basic Med Sci 2016;19:794–9. https://doi.org/10.22038/ijbms.2016.7366.
Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 2017;17:545–58. https://doi.org/10.1038/nri.2017.52.
Tang X, Li Q, Huang T, Zhang H, Chen X, Ling J, et al. Regenerative Role of T Cells in Nerve Repair and Functional Recovery. Front Immunol 2022;13:1–7. https://doi.org/10.3389/fimmu.2022.923152.
Quan S ming, Zhi-qiang G. Immunobiology of Facial Nerve Repair and Regeneration. J Otol 2006;1:107–15. https://doi.org/10.1016/S1672-2930(06)50023-6.
Guida MS, El-Aal AA, Kafafy Y, Salama SF, Badr BM, Badr G. Thymoquinone rescues T lymphocytes from gamma irradiation-induced apoptosis and exhaustion by modulating pro-inflammatory cytokine levels and PD-1, bax, and Bcl-2 signaling. Cell Physiol Biochem 2016;38:786–800. https://doi.org/10.1159/000443034.
Shaterzadeh-Yazdi H, Noorbakhsh M-F, Hayati F, Samarghandian S, Farkhondeh T. Immunomodulatory and Anti-inflammatory Effects of Thymoquinone. Cardiovasc Hematol Disord Targets 2018;18:52–60. https://doi.org/10.2174/1871529x18666180212114816.
Acknowledgment
The authors would like to acknowledge the staff at the Institute of Tropical Disease, Department of Clinical Pathology and Department of Biochemistry, Faculty of Medicine, Airlangga University, for their technical assistance during this study. This research was additionally guided by the late Professor Mohammad Hasan Machfoed, MD of the Department of Neurology, Faculty of Medicine, Airlangga University in Surabaya, Indonesia.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Valentinus Besin and Abdul Hafid Bajamal. The first draft of the manuscript was written by Valentinus Besin and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to publication
This study was published with the informed consent of all authors
Ethical approval
The study underwent a rigorous review from the Health Research Ethics Committee of the Faculty of Medicine, Airlangga University, Surabaya approved the protocol for this study (4/EC/KEPK/FKUA/2021).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besin, V., Bajamal, A.H., Nugraha, J. et al. Thymoquinone modifies CD4+:CD8+ ratio without affecting tumor necrosis factor-α and interleukin-1β levels in Wallerian degeneration crush injury rat model. Neurosci Behav Physi 54, 1–9 (2024). https://doi.org/10.1007/s11055-023-01523-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01523-4