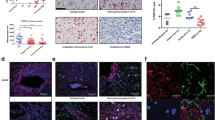
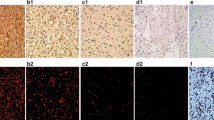
Objectives. To study the relationship between the expression activity of NMDA glutamate receptors and the proliferative activity and genetic properties of anaplastic astrocytomas, as well as the survival of patients with this disease. Materials and methods. This task was addressed by comparing the expression activity of NMDA glutamate receptors – which have been least studied in this context – as detected by immunofluorescence and the polymerase chain reaction, with data from histological and molecular studies, the proliferative activity of neoplasms, and patient survival. Results. The expression activity of NMDA glutamate receptors was found to be higher in grade 3 astrocytomas not carrying mutations in the IDH1 and IDH2 genes. NMDA receptor expression activity was also found to correlate directly with proliferative activity in tumors. NMDA receptor expression activity had a significant impact on the prognosis of relapse-free survival in patients. Conclusions. This work provides the first demonstration that NMDA glutamate receptors have a significant role in the progression of diffuse astrocytomas, which can become the basis for the creation of new approaches to treatment and diagnosis.
Similar content being viewed by others
References
T. R. Izmailov, G. L. Kobyakov, S. M. Banov, et al., Primary Tumors of the Central Nervous System. Clinical Guidelines, Russian Association of Neurosurgeons, Moscow (2020).
Q. T. Ostrom, N. Patil, G. Cioffi, et al., “CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017,” Neuro. Oncol., 22, No. 12, Suppl. 2, 1–96 (2020), https://doi.org/10.1093/neuonc/noaa200.
Q. T. Ostrom, H. Gittleman, G. Truitt, et al., “CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015,” Neuro. Oncol., 20, Suppl. 4, 1–86 (2018), https://doi.org/https://doi.org/10.1093/neuonc/noy131.
G. L. Kobyakov, O. V. Absalyamova, O. Yu. Anikeeva, et al., “Practical guidelines for drug treatment of primary tumors of the central nervous system,” Zlokachestv. Opukh., 4, 55–79 (2015), https://doi.org/10.18027/2224-5057-2020-10-3s2-07.
E. A. Kobyakova, D. Yu. Usachev, O. V. Absalyamova, et al., “Criteria for assessing treatment responses in neuro-oncology (RANO): application in clinical trials and routine practice,” Farmateka, 28, No. 11, 21–33 (2021), https://doi.org/10.18565/pharmateca.2021.11.21-33.
M. J. van den Bent, M. Smits, J. M. Kros, et al., “Diffuse infiltrating oligodendroglioma and astrocytoma,” J. Clin. Oncol., 35, No. 21, 2394–2401 (2017), https://doi.org/https://doi.org/10.1200/JCO.2017.72.6737.
M. Caccese, M. Padovan, D. D’Avella, et al., “Anaplastic astrocytoma: State of the art and future directions,” Crit. Rev. Oncol. Hematol., 153, 103062 (2020), https://doi.org/https://doi.org/10.1016/j.critrevonc.2020.103062.
H. S. Venkatesh, W. Morishita, A. C. Geraghty, et al., “Electrical and synaptic integration of glioma into neural circuits,” Nature, 573, No. 7775, 539–545 (2019), https://doi.org/https://doi.org/10.1038/s41586-019-1563-y.
V. Venkataramani, D. I. Tanev, C. Strahle, et al., “Glutamatergic synaptic input to glioma cells drives brain tumour progression,” Nature, 573, No. 7775, 532–538 (2019), https://doi.org/https://doi.org/10.1038/s41586-019-1564-x.
T. G. Smart and People, Paoletti, “Synaptic neurotransmitter-gated receptors,” Cold Spring Harb. Perspect. Biol., 4, No. 3, a009662 (2012), https://doi.org/10.1101/cshperspect.a009662.
C. Lüscher and R. C. Malenka, “NMDA receptor-dependent longterm potentiation and long-term depression (LTP/LTD),” Cold Spring Harb. Perspect. Biol., 4, No. 6, a005710 (2012), https://doi.org/10.1101/cshperspect.a005710.
V. Durante, A. de Iure, V. Loffredo, et al., “Alpha-synuclein targets GluN2A NMDA receptor subunit causing striatal synaptic dysfunction and visuospatial memory alteration,” Brain, 142, No. 5, 1365–1385 (2019), https://doi.org/https://doi.org/10.1093/brain/awz065.
V. P. Nikitin, S. A. Kozyrev, S. V. Solntseva, et al., “Protein synthesis inhibitor administration before a reminder caused recovery from amnesia induced by memory reconsolidation impairment with NMDA glutamate receptor antagonist,” Brain Res. Bull., 171, 44–55 (2021), https://doi.org/https://doi.org/10.1016/j.brainresbull.2021.03.008.
P. Ramaswamy, N. N. Dalavaikodihalli, C. Prasad, et al., “Transcriptional modulation of calcium-permeable AMPA receptor subunits in glioblastoma by MEK-ERK1/2 inhibitors and their role in invasion,” Cell Biol. Int., 44, No. 3, 830–837 (2020), https://doi.org/https://doi.org/10.1002/cbin.11279.
Z. Pei, K. C. Lee, A. Khan, et al., “Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression,” Biochem. Pharmacol., 176, 113814 (2020), https://doi.org/https://doi.org/10.1016/j.bcp.2020.113814.
L. Corsi, A. Mescola, and Alessandrini, A., “Glutamate receptors and glioblastoma multiforme: An old ‘route’ for new perspectives,” Int. J. Mol. Sci., 20, No. 7, 1796 (2019), https://doi.org/10.3390/ijms20071796.
V. Venkataramani, D. I. Tanev, T. Kuner, et al., “Synaptic input to brain tumors: clinical implications,” Neuro. Oncol., 23, No. 1, 23–33 (2021), https://doi.org/https://doi.org/10.1093/neuonc/noaa158.
D. S. Gryder, D. C. Castaneda, and M. A. Rogawski, “Evidence for low GluR2 AMPA receptor subunit expression at synapses in the rat basolateral amygdala,” J. Neurochem., 94, No. 6, 1728–1738 (2005), https://doi.org/https://doi.org/10.1111/j.1471-4159.2005.03334.x.
J. Mayer, T. Kirschstein, T. Resch, et al., “Perampanel attenuates epileptiform phenotype in C6 glioma,” Neurosci. Lett., 715,134629 (2020), https://doi.org/https://doi.org/10.1016/j.neulet.2019.134629.
M. Mittelbronn, P. Harter, A. Warth, et al., “EGR-1 is regulated by N-methyl-D-aspartate-receptor stimulation and associated with patient survival in human high grade astrocytomas,” Brain Pathol., 19, No. 2, 195–204 (2009), https://doi.org/https://doi.org/10.1111/j.1750-3639.2008.00175.x.
H. S. Chen and S. A. Lipton, “The chemical biology of clinically tolerated NMDA receptor antagonists,” J. Neurochem., 97, No. 6, 1611–1626 (2006), https://doi.org/https://doi.org/10.1111/j.1471-4159.2006.03991.x.
W. Limapichat, W. Y. Yu, E. Branigan, et al., “Key binding interactions for memantine in the NMDA receptor,” ACS Chem. Neurosci., 4, No. 2, 255–260, https://doi.org/https://doi.org/10.1021/cn300180a. (2013).
H. Lavretsky, K. T. Laird, B. Krause-Sorio, et al., “A randomized double-blind placebo-controlled trial of combined escitalopram and memantine for older adults with major depression and subjective memory complaints,” Am. J. Geriatr. Psychiatry, 28, No. 2, 178–190 (2020), https://doi.org/https://doi.org/10.1016/j.jagp.2019.08.011.
D. N. Louis, A. Perry, G. Reifenberger, et al., “The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary,” Acta Neuropathol., 131, No. 6, 803–820 (2016), https://doi.org/https://doi.org/10.1007/s00401-016-1545-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 123, No. 1, Iss. 1, pp. 97–102, January, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikitin, P.V., Belyaev, A.Y., Kobyakov, G.L. et al. NMDA Glutamate Receptor Expression Activity in Anaplastic Astrocytomas. Neurosci Behav Physi 53, 1114–1119 (2023). https://doi.org/10.1007/s11055-023-01508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01508-3