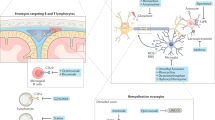
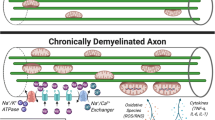
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS), which leads to the formation of foci of primary demyelination in the white and gray matter and diffuse damage to axons and neurons. Despite significant progress in the development of drugs for relapsing-remitting MS, the impact on the progression of the disease remains insufficient. Diffuse and compartmentalized lymphocyte and macrophage infiltration of CNS tissue inhibits the differentiation of myelinating mature oligodendrocytes and disrupts remyelination processes. Chronic inflammation, which occurs when the blood–brain barrier (BBB) is intact, activates microglia, increasing axon and neuron damage and, as a result, triggers chronic oxidative stress and histotoxic hypoxia. It is currently important to clarify the mechanisms underlying neurodegeneration, which in the later stages of MS is caused by chronic neuroaxonal damage and impairment of regenerative capabilities and which largely determines disease outcome.
Similar content being viewed by others
References
H. Lassmann, W. Brück, and C. Lucchinetti, “The immunopathology of multiple sclerosis: an overview,” Brain Pathol., 17, No. 2, 210–218 (2007), https://doi.org/10.1111/j.1750-3639.2007.00064.x.
M. Adamczyk-Sowa, B. Adamczyk, A. Kułakowska, et al., “Secondary progressive multiple sclerosis – from neuropathology to definition and effective treatment,” Neurol. Neurochir. Pol., 54, No. 5, 384–398 (2020), https://doi.org/10.5603/PJNNS.a2020.0082.
B. Weinshenker, D. Reich, C. Lucchinetti, et al., “The natural history of multiple sclerosis: a geographically based study,” New Engl. J. Med., 378, 169–180 (2018), https://doi.org/10.1093/brain/112.1.133.
F. D. Lublin, S. C. Reingold, J. A. Cohen, et al., “Defining the clinical course of multiple sclerosis: the 2013 revisions,” Neurology, 83, No. 3, 278–286 (2014), https://doi.org/10.1212/WNL.0000000000000560.
R. Hohlfeld, K. Dornmair, E. Meinl, et al., “The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets,” Lancet Neurol., 15, No. 2, 198–209 (2016), https://doi.org/10.1016/S1474-4422(15)00334-8.
B. D. Trapp and K. Nave, “Multiple sclerosis: an immune or neurodegenerative disorder?,” Annu. Rev. Neurosci., 31, 247–269 (2008), https://doi.org/10.1146/annurev.neuro.30.051606.094313.
P. Stys, G. Zamponi, J. van Minnen, et al., “Will the real multiple sclerosis please stand up?” Nat. Rev. Neurosci., 13, No. 7, 507–514 (2012), https://doi.org/10.1038/nrn3275.
C. Confavreux and S. Vukusi, “Natural history of multiple sclerosis: a unifying concept,” Brain, 129, No. 3, 606–616 (2006), https://doi.org/10.1093/brain/awl007.
M. Novotna, M. Paz Soldán, N. Abou Zeid, et al., “Poor early relapse recovery affects onset of progressive disease course in multiple sclerosis,” Neurology, 85, No. 8, 722–729 (2015), https://doi.org/10.1212/WNL.0000000000001856.
A. Scalfari, A. Neuhaus, A. Degenhard, et al., “The natural history of multiple sclerosis: a geographically based study 10,” Brain, 133, No. 7, 1914–1929 (2010), https://doi.org/10.1093/brain/awq118.
H. Yong and V. W. Yong, “Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis,” Nat. Rev. Neurol., 18, No. 1, 40–55 (2022), https://doi.org/10.1038/s41582-021-00581-x.
M. Sádaba, J. Tzartos, C. Paíno, et al., “Axonal and oligodendrocytelocalized IgM and IgG deposits in MS lesions,” J. Neuroimmunol., 247, No. 1–2, 86–94 (2012), https://doi.org/10.1016/j.jneuroim.2012.03.020.
T. Kuhlmann, S. Ludwin, A. Prat, et al., “An updated histological classification system for multiple sclerosis lesions,” Acta Neuropathol., 133, No. 1, 13–24 (2017), https://doi.org/10.1007/s00401-016-1653-y.
J. Frischer, S. Weigand, Y. Guo, et al., “Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque,” Ann. Neurol., 78, No. 5, 710–721 (2015), https://doi.org/10.1002/ana.24497.
D. H. Mahad, B. D. Trapp, and H. Lassmann, “Pathological mechanisms in progressive multiple sclerosis,” Lancet Neurology, 14, No. 2, 183–193 (2015), https://doi.org/10.1016/S1474-4422(14)70256-X.
S. L. Hauser and J. R. Oksenberg, “The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration,” Neuron, 52, No. 1, 61–76 (2006), https://doi.org/10.1016/j.neuron.2006.09.011.
H. Kebir, K. Kreymborg, I. Ifergan, et al., “Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation,” Nat. Med., 13, No. 10, 1173–1175 (2007), https://doi.org/10.1038/nm1651.
G. P. van Nierop, M. M. van Luijn, S. S. Michels, et al., “Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients,” Acta Neuropathol., 134, No. 3, 383–401 (2017), https://doi.org/10.1007/s00401-017-1744-4.
T. Mockus, A. Munie, J. Atkinson, et al., “Encephalitogenic and regulatory CD8 T cells in multiple sclerosis and its animal models,” J. Immunol., 206, No. 1, 3–10 (2021), https://doi.org/10.4049/jimmunol.2000797.
S. Na, A. Hermann, M. Sanchez-Ruiz, et al., “Oligodendrocytes enforce immune tolerance of the uninfected brain by purging the peripheral repertoire of autoreactive CD8+ T cells,” Immunity, 37, No. 1, 134–146 (2012), https://doi.org/10.1016/j.immuni.2012.04.009.
H. Neumann, I. Medana, J. Bauer, et al., “Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases,” Trends Neurosci., 25, No. 6, 313–319 (2002), https://doi.org/10.1016/s0166-2236(02)02154-9.
J. Tzartos, M. Friese, M. Craner, et al., “Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis,” Am. J. Pathol., 172, No. 1, 146–155 (2008), https://doi.org/10.2353/ajpath.2008.070690.
R. Lisak, J. Benjamins, L. Nedelkoska, et al., “Secretory products of multiple sclerosis B cells cytotoxic to oligodendroglia in vitro,” J. Neuroimmunol., 246, No. 1–2, 85–95 (2012), https://doi.org/10.1016/j.jneuroim.2012.02.015.
J. Machado-Santos, E. Saji, A. Tröscher, et al., “The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells,” Brain, 141, No. 7, 2066–2082 (2018), https://doi.org/10.1093/brain/awy151.
A. A. Abramova, I. V. Zakroyshchikova, I. A. Krotenkova, et al., “Leptomeningeal B-cell follicles in multiple sclerosis: a role in the pathogenesis and prognostic value,” Zh. Nevrol. Psikhiat., 119, No. 10, Part 2, 21–27 (2019), https://doi.org/10.17116/jnevro20191191021.
D. M. Harrison, K. Wang, J. Fiol, et al., “Leptomeningeal enhancement at 7T in multiple sclerosis: Frequency, morphology, and relationship to cortical volume,” J. Neuroimaging, 27, No. 5, 461–468 (2017), https://doi.org/10.1111/jon.12444.
H. Lassmann, “Pathogenic mechanisms associated with different clinical courses of multiple sclerosis,” Front. Immunol., 9, 3116–3119 (2019), https://doi.org/10.3389/fimmu.2018.03116.
R. Magliozzi, O. Howell, A. Vora, et al., “Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology,” Brain, 130, No. 4, 1089–1104 (2007), https://doi.org/10.1093/brain/awm038.
O. W. Howell, C. A. Reeves, R. Nicholas, et al., “Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis,” Brain, 134, No. 9, 2755–2771 (2011), https://doi.org/10.1093/brain/awr182.
F. Aloisi, B. Serafini, R. Magliozzi, et al., “Detection of Epstein–Barr virus and B-cell follicles in the multiple sclerosis brain: what you find depends on how and where you look,” Brain, 133, No. 12, e157 (2010), https://doi.org/10.1093/brain/awr221.
B. Serafini, B. Rosicarelli, R. Magliozzi, et al., “Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis,” Brain Pathol., 14, 164–174 (2004), https://doi.org/10.1111/j.1750-3639.2004.tb00049.x.
M. Mitsdoerffer and A. Peters, “Tertiary lymphoid organs in central nervous system autoimmunity,” Front. Immunol., 7, 451 (2016), https://doi.org/10.3389/fimmu.2016.00451.
C. Veroni, B. Serafini, B. Rosicarelli, et al., “Transcriptional profile and Epstein Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis,” J. Neuroinflammation, 15, 18 (2018), https://doi.org/10.1186/s12974-017-1049-5.
A. Ascherio and K. Munger, “Epidemiology of multiple sclerosis: From risk factors to prevention-an update,” Semin. Neurol., 36, No. 2, 103–114 (2016), https://doi.org/10.1055/s-0036-1579693.
C. Reali, R. Magliozzi, F. Roncaroli, et al., “B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis,” Brain Pathol., 30, No. 4, 779–793 (2020), https://doi.org/10.1111/bpa.12841.
S. Choi, O. Howell, D. Carassiti, et al., “Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis,” Brain, 135, No. 10, 2925–2937 (2012), https://doi.org/10.1093/brain/aws189.
M. Calabrese, F. Agosta, F. Rinaldi, et al., “Cortical lesions and atrophy associated with cognitive impairment in multiple sclerosis,” Arch. Neurol., 66, No. 9, 1144–1150 (2009), https://doi.org/10.1001/archneurol.2009.174.
L. Bø, C. Vedeler, H. Nyland, et al., “Subpial demyelination in the cerebral cortex of multiple sclerosis patients,” J. Neuropathol. Exp. Neurol., 62, No. 7, 723–732 (2003), https://doi.org/10.1093/jnen/62.7.723.
D. Harrison, S. Roy, J. Oh, et al., “Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis,” JAMA Neurol., 72, No. 9, 1004–1012 (2015), https://doi.org/10.1001/jamaneurol.2015.1241.
A. Kutzelnigg, C. Lucchinetti, C. Stadelmann, et al., “Cortical demyelination and diffuse white matter injury in multiple sclerosis,” Brain, 128, No. 11, 2705–2712 (2005), https://doi.org/10.1093/brain/awh641.
C. F. Lucchinetti, B. F. Popescu, R. F. Bunyan, et al., “Inflammatory cortical demyelination in early multiple sclerosis,” New Engl. J. Med., 365, 2188–2197 (2011), https://doi.org/10.1056/NEJMoa1100648.
K. Blauth, J. Soltys, A. Matschulat, et al., “Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid cause demyelination of spinal cord explants,” Acta Neuropathol., 130, No. 6, 765–781 (2015), https://doi.org/10.1007/s00401-015-1500-6.
R. Chu, S. Hurwitz, S. Tauhid, et al., “Automated segmentation of cerebral deep gray matter from MRI scans: effect of field strength on sensitivity and reliability,” BMC Neurol., 17, No. 1, 172 (2017), https://doi.org/10.1186/s12883-017-0949-4.
L. Haider, C. Simeonidou, G. Steinberger, et al., “Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron,” J. Neurol. Neurosurg. Psychiatr., 85, No. 12, 1386–1395 (2014), https://doi.org/10.1136/jnnp-2014-307712.
K. Nave and H. Werner, “Myelination of the nervous system: mechanisms and functions,” Annu. Rev. Cell. Dev. Biol., 30, 503–533 (2014), https://doi.org/10.1146/annurev-cellbio-100913-013101.
R. Franklin and S. Goldman, “Glia disease and repair-remyelination,” Cold Spring Harb. Perspect. Biol., 7, No. 7, a020594 (2015), https://doi.org/10.1101/cshperspect.a020594.
S. Mitew, C. Hay, H. Peckham, et al., “Mechanisms regulating the development of oligodendrocytes and central nervous system myelin,” Neurosci., 276, 29–47 (2014), https://doi.org/10.1016/j.neuroscience.2013.11.029.
L. Clarke, K. Young, N. Hamilton, et al., “Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse,” J. Neurosci., 32, 8173–8185 (2012), https://doi.org/10.1523/jneurosci.0928-12.2012.
D. McTigue, P. Wei, and B. Stokes, “Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord,” J. Neurosci., 21, No. 10, 3392–3400 (2001), https://doi.org/10.1523/JNEUROSCI.21-10-03392.2001.
F. Birey, M. Kloc, M. Chavali, et al., “Genetic and Stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2,” Neuron, 88, No. 5, 941–956 (2015), https://doi.org/10.1016/j.neuron.2015.10.046.
E. Hughes, S. Kang, M. Fukaya, et al., “Oligodendrocyte progenitors balance growth self-repulsion to achieve homeostasis adult brain,” Nat. Neurosci., 16, No. 6, 668–676 (2013), https://doi.org/10.1038/nn.3390.
M. Zawadzka, L. Rivers, S. Fancy, et al., “CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination,” Cell Stem Cell, 6, No. 6, 578–590 (2010), https://doi.org/10.1016/j.stem.2010.04.002.
T. Kuhlmann, V. Miron, Q. Cui, et al., “Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis,” Brain, 131, No. 7, 1749–1758 (2008), https://doi.org/10.1093/brain/awn096.
A. Foote and F. Blakemore, “Inflammation stimulates remyelination in areas of chronic demyelination,” Brain, 128, No. 3, 528–539 (2005), https://doi.org/10.1093/brain/awh417.
S. Mi, R. Miller, W. Tang, et al., “Promotion of central nervous system remyelination by differentiation of oligodendrocyte precursor cells,” Ann. Neurol., 65, No. 3, 304–315 (2009), https://doi.org/10.1002/ana.21581.
P. Patrikios, C. Stadelmann, A. Kutzelnigg, et al., “Remyelination is extensive in a subset of multiple sclerosis patients,” Brain, 129, No. 12, 3165–3172 (2006), https://doi.org/10.1093/brain/awl217.
E. D. Ponomarev, L. P. Shriver, K. Maresz, et al., “Microglial cell activation and proliferation precedes the onset of CNS autoimmunity,” J. Neurosci. Res., 81, No. 3, 374–389 (2005), https://doi.org/10.1002/jnr.20488.
J. Xue, S. Schmidt, J. Sander, et al., “Transcriptome-based network analysis reveals a spectrum model of human macrophage activation,” Immunity, 40, No. 2, 274–288 (2014), https://doi.org/10.1016/j.immuni.2014.01.006.
E. Benveniste, “Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis,” J. Mol. Med. (Berl.), 75, No. 3, 165–173 (1997), https://doi.org/10.1007/s001090050101.
E. Frohman, M. Racke, and C. Raine, “Multiple sclerosis – the plaque and its pathogenesis,” New Engl. J. Med., 354, No. 9, 942–955 (2006), https://doi.org/10.1056/NEJMra052130.
M. Prinz and J. Priller, “Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease,” Nat. Rev. Neurosci., 15, No. 5, 300–312 (2014), 10.1038nrn3722.
E. Miller, B. Wachowicz, and I. Majsterek, “Advances in antioxidative therapy of multiple sclerosis,” Curr. Med. Chem., 20, No. 37, 4720–4730 (2013), https://doi.org/10.2174/09298673113209990156.
M. Fischer, R. Sharma, J. Lim, et al., “NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage,” Brain, 135, No. 3, 886–899 (2012), https://doi.org/10.1093/brain/aws012.
L. Haider, M. Fischer, J. Frischer, et al., “Oxidative damage in multiple sclerosis lesions,” Brain, 134, No. 7, 1914–1924 (2011), https://doi.org/10.1093/brain/awr128.
J. Correale and M. Farez, “The Role of astrocytes in multiple sclerosis progression,” Front. Neurol., 6, 180 (2015), https://doi.org/10.3389/fneur.2015.00180.
A. Argaw, L. Asp, J. Zhang, et al., “Astrocyte-derived VEGF-A drives blood–brain barrier disruption in CNS inflammatory disease,” J. Clin. Invest., 122, No. 7, 2454–2468 (2012), https://doi.org/10.1172/JCI60842.
M. Gimenez, J. Sim, and J. Russell, “TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to inflammation,” J. Neuroimmunol., 151, No. 1–2, 116–125 (2004), https://doi.org/10.1016/j.jneuroim.2004.02.012.
Y. Wang, X. Cheng, Q. He, et al., “Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins,” J. Neurosci., 31, No. 16, 6053–6058 (2011), https://doi.org/10.1523/JNEUROSCI.5524-09.2011.
K. Omari, G. John, S. Sealfon, et al., “CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis,” Brain, 128, No. 5, 1003–1015 (2005), https://doi.org/10.1093/brain/awh479.
C. Luo, C. Jian, Y. Liao, et al., “The role of microglia in multiple sclerosis,” Neuropsychiatr. Dis. Treat., 13, 1661–1667 (2017), https://doi.org/10.2147/NDT.S140634.
K. Smith, R. Kapoor, and P. Felts, “Demyelination: the role of reactive oxygen and nitrogen species,” Brain Pathol., 9, No. 1, 69–92 (1999), https://doi.org/10.1111/j.1750-3639.1999.tb00212.x.
O. Bizzozero, G. DeJesus, K. Callahan, et al., “Elevated protein carbonylation in brain white and gray matter patients multiple sclerosis,” J. Neurosci. Res., 81, No. 5, 687–695 (2005), https://doi.org/10.1002/jnr.20587.
B. Butts, C. Houde, and H. Mehmet, “Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: implications for normal development and disease,” Cell Death Differ., 15, No. 7, 1178–1186 (2008), https://doi.org/10.1038/cdd.2008.70.
K. Blomgren and H. Hagberg, “Free radicals, mitochondria, and hypoxia-ischemia in the developing brain,” Free Radic. Biol. Med., 40, No. 3, 388–397 (2006), https://doi.org/10.1016/j.freeradbiomed.2005.08.040.
B. Trapp and P. Stys, “Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis,” Lancet Neurol., 8, No. 3, 280–291 (2009), https://doi.org/10.1016/S1474-4422(09)70043-2.
N. Lee, S. Ha, P. Sati, et al., “Potential role of iron in repair of inflammatory demyelinating lesions,” J. Clin. Invest., 129, No. 10, 4365–4376 (2019), https://doi.org/10.1172/JCI126809.
S. Cronin, C. Woolf, G. Weiss, et al., “The role of iron regulation in immunometabolism and immune-related disease,” Front. Mol. Biosci., 6, 116–120 (2019), https://doi.org/10.3389/fmolb.2019.00116.
P. Urrutia, P. Aguirre, A. Esparza, et al., “Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells,” J. Neurochem., 126, No. 4, 541–549 (2013), https://doi.org/10.1111/jnc.12244.
S. Hametner, I. Wimmer, L. Haider, et al., “Iron and neurodegeneration in the multiple sclerosis brain,” Ann. Neurol., 74, No. 6, 848–861 (2013), https://doi.org/10.1002/ana.23974.
F. Bagnato, S. Hametner, B. Yao, et al., “Tracking iron in multiple sclerosis: a combined study at 7 Tesla,” Brain, 134, No. 12, 3602–3615 (2011), https://doi.org/10.1093/brain/awr278.
M. Filippi, W. Brück, D. Chard, et al., attendees of the Correlation between Pathological and MRI findings in MS workshop (2019), “Association between pathological and MRI findings in multiple sclerosis,” Lancet Neurol., 18, No. 2, 198–210 (2019), https://doi.org/10.1016/S1474-4422(18)30451-4.
A. Dal-Bianco, G. Grabner, C. Kronnerwetter, et al., “Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI,” Brain, 144, No. 3, 833–847 (2021), https://doi.org/10.1093/brain/awaa436.
A. Elkady, D. Cobzas, H. Sun, et al., “Progressive iron accumulation across multiple sclerosis phenotypes classification of deep gray matter,” J. Magn. Reson. Imaging, 46, No. 5, 1464–1473 (2017), https://doi.org/10.1002/jmri.25682.
N. Bergsland, E. Tavazzi, M. Laganà, et al., “White matter tract injury is associated with deep gray matter iron deposition in multiple sclerosis,” J. Neuroimaging, 27, No. 1, 107–113 (2017), https://doi.org/10.1111/jon.12364.
R. Zivadinov, E. Tavazzi, N. Bergsland, et al., “Brain iron at quantitative MRI is associated with disability in multiple sclerosis,” Radiology, 289, No. 2, 487–496 (2018), https://doi.org/10.1148/radiol.2018180136.
H. Wiendl, R. Gold, T. Berger, et al., “Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper),” Ther. Adv. Neurol. Disord., 14, 17562864211039648 (2021), https://doi.org/10.1177/17562864211039648.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 122, No. 7, Iss. 2, pp. 5–13, July, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eliseeva, D.D., Zakharova, M.N. Mechanisms of Neurodegeneration in Multiple Sclerosis. Neurosci Behav Physi 53, 324–332 (2023). https://doi.org/10.1007/s11055-023-01429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01429-1