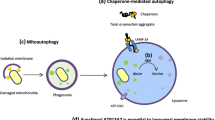
Genetic factors underlie the pathological processes responsible for the manifestations of a wide range of neurodegenerative diseases. The expansion of unstable trinucleotide repeats forms pathological alleles that lead to the development of monogenic neurological diseases (Huntington’s disease, Kennedy’s disease, spinocerebellar ataxia, etc.). However, data have recently appeared on the role of alleles with intermediate numbers of these repeats in the formation of the clinical features of multifactorial neurological phenotypes (Parkinson’s disease, Alzheimer’s disease). This article summarizes data on current concepts of the role of the “intermediate” allele, mainly of the huntingtin gene (HTT), in the pathogenesis and clinical picture of neurodegenerative diseases, primarily Parkinson’s disease. The issue of the need to develop a special tactic for managing individual carriers of the “intermediate” allele by teams of highly qualified specialists in neurology and genetics is also discussed, as this would allow neurodegenerative diseases to be diagnosed early and medical genetic counseling of families to be improved.
Similar content being viewed by others
References
A. J. Hannan, “Tandem repeats and repeatomes: Delving deeper into the ‘dark matter’ of genomes,” EBioMedicine, 31, 3–4 (2018), https://doi.org/10.1016/j.ebiom.2018.04.004.
A. J. Hannan, “Tandem repeat polymorphisms: Mediators of genetic plasticity, modulators of biological diversity and dynamic sources of disease susceptibility,” Adv. Exp. Med. Biol., 769, 1–9 (2012).
C. M. Rodriguez and P. K. Todd, “New pathologic mechanisms in nucleotide repeat expansion disorders,” Neurobiol. Dis., 130, 104515 (2019), https://doi.org/10.1016/j.nbd.2019.104515.
H. Paulson, “Repeat expansion diseases,” Handb. Clin. Neurol., 147, 105–123 (2018), https://doi.org/10.1016/B978-0-444-63233-3.00009-9.
S. L. Gardiner, M. W. Boogaard, S. Trompet, et al., “Prevalence of carriers of intermediate and pathological polyglutamine disease-associated alleles among large population-based cohorts,” JAMA Neurol., 76, No. 6, 650–656 (2019), https://doi.org/10.1001/jamaneurol.2019.0423.
G. P. Bates, R. Dorsey, J. F. Gusella, et al., “Huntington disease,” Nat. Rev. Dis. Primers, 1, 15005 (2015), https://doi.org/10.1038/nrdp.2015.5.
A. Killoran, K. M. Biglan, J. Jankovic, et al., “Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS,” Neurology, 80, No. 22, 2022–2027 (2013), https://doi.org/10.1212/WNL.0b013e318294b304.
P. Hogarth, “Huntington disease: How many repeats does it take?” Neurology, 80, No. 22, e241–243 (2013), https://doi.org/10.1212/WNL.0b013e3182984b31.
M. Menéndez-González, J. Clarimón, I. Rosas-Allende, et al., “HTT gene intermediate alleles in neurodegeneration: evidence for association with Alzheimer’s disease,” Neurobiol. Aging, 76, 215.e9–215.e14 (2019), https://doi.org/10.1016/j.neurobiolaging.2018.11.014.
I. Rosas, C. Martínez, J. Clarimón, et al., “Role for ATXN1, ATXN2, and HTT intermediate repeats in frontotemporal dementia and Alzheimer’s disease,” Neurobiol. Aging, 87, 139.e1–139.e7 (2020), https://doi.org/10.1016/j.neurobiolaging.2019.10.017.
V. Bessi, S. Mazzeo, S. Bagnoli, et al., “The effect of CAG repeats within the non-pathological range in the HTT gene on cognitive functions in patients with subjective cognitive decline and mild cognitive impairment,” Diagnostics (Basel), 11, No. 6, 1051 (2021), https://doi.org/10.3390/diagnostics11061051.
J. K. Lee, Y. Ding, A. L. Conrad, et al., “Sex-specific effects of the Huntington gene on normal neurodevelopment,” J. Neurosci. Res., 95, No. 1–2, 398–408 (2017), https://doi.org/10.1002/jnr.23980.
M. Mühlau, J. Winkelmann, D. Rujescu, et al., “Variation within the Huntington’s disease gene infl uences normal brain structure,” PLoS One, 7, No. 1, e29809 (2012), https://doi.org/10.1371/journal.pone.0029809.
V. Bessi, S. Mazzeo, S. Bagnoli, et al., “The implication of BDNF Val66Met polymorphism in progression from subjective cognitive decline to mild cognitive impairment and Alzheimer’s disease: a 9-year follow-up study,” Eur. Arch. Psychiatry Clin. Neurosci., 270, No. 4, 471–482 (2020), https://doi.org/10.1007/s00406-019-01069-y.
P. A. Ong, F. R. Annisafitrie, N. Purnamasari, et al., “dementia prevalence, comorbidities, and lifestyle among Jatinangor elders,” Front. Neurol., 12, 643480 (2021), https://doi.org/10.3389/fneur.2021.643480.
A. J. Hannan, “Tandem repeats mediating genetic plasticity in health and disease,” Nat. Rev. Genet., 19, No. 5, 286–298 (2018), https://doi.org/10.1038/nrg.2017.115.
V. Zabnenkova, O. A. Schagina, N. M. Galeeva, et al., “HTT gene premutation allele frequencies in the Russian Federation,” Russ. J. Genet., 54, 732–739 (2018), https://doi.org/10.1134/S1022795418060169.
A. J. Semaka, Genetic Counselling Implications for Intermediate Allele Predictive Test Results for Huntington Disease, University of British Columbia (2012), https://open.library.ubc.ca/collections/ubctheses/24/items/1.0071843.
M. I. Alvarez-Mora, I. Madrigal, F. Martinez, et al., “Clinical implication of FMR1 intermediate alleles in a Spanish population,” Clin. Genet., 94, No. 1, 153–158 (2018), https://doi.org/10.1111/cge.13257.
H. L. Paulson, V. G. Shakkottai, H. B. Clark, and H. T. Orr, “Polyglutamine spinocerebellar ataxias – from genes to potential treatments,” Nat. Rev. Neurosci., 18, No. 10, 613–626 (2017), https://doi.org/10.1038/nrn.2017.92.
S. Lattante, M. G. Pomponi, A. Conte, et al., “ATXN1 intermediatelength polyglutamine expansions are associated with amyotrophic lateral sclerosis,” Neurobiol. Aging, 64, 157.e1–157.e5 (2018), https://doi.org/10.1016/j.neurobiolaging.2017.11.011.
P. Van Damme, J. H. Veldink, M. van Blitterswijk, et al., “Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2,” Neurology, 76, No. 24, 2066–2072 (2011), https://doi.org/10.1212/WNL.0b013e31821f445b.
T. Lee, Y. R. Li, A. Chesi, et al., “Evaluating the prevalence of polyglutamine repeat expansions in amyotrophic lateral sclerosis,” Neurology, 76, No. 24, 2062–2065 (2011), https://doi.org/10.1212/WNL.0b013e31821f4447.
L. Choubtum, P. Witoonpanich, K. Kulkantrakorn, et al., “Trinucleotide repeat expansion of TATA-binding protein gene associated with Parkinson’s disease: A Thai multicenter study,” Parkins. Relat. Disord., 28, 146–149 (2016), https://doi.org/10.1016/j.parkreldis.2016.05.008.
M. B. Hammer and A. B. Singleton, “Common premutations in the general population,” JAMA Neurol., 76, No. 6, 639–640 (2019), https://doi.org/10.1001/jamaneurol.2019.0216.
D. Z. Loesch, M. S. Khaniani, H. R. Slater, et al., “Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism,” Clin. Genet., 76, No. 5, 471–476 (2009), Epub Sep. 30, 2009, PMCID: PMC2888465, PMID: 19796183, https://doi.org/10.1111/j.1399-0004.2009.01275.x.
M. W. Kurz, A. M. Schlitter, Y. Klenk, et al., “FMR1 alleles in Parkinson’s disease: relation to cognitive decline and hallucinations, a longitudinal study,” J. Geriatr. Psychiatry Neurol., 20, No. 2, 89–92 (2007), https://doi.org/10.1177/0891988706297737.
D. Z. Loesch, F. Tassone, G. D. Mellick, et al., “Evidence for the role of FMR1 gray zone alleles as a risk factor for parkinsonism in females,” Mov. Disord., 33, No. 7, 1178–1181 (2018), PMCID: PMC6116531, PMID: 30153395, https://doi.org/10.1002/mds.27420.
D. A. Hall, “In the gray zone in the fragile X gene: What are the key unanswered clinical and biological questions?”. Tremor Other Hyperkinet Mov (NY), 4, 208 (2014), https://doi.org/10.7916/D8NG4NP3.
L. N. Clark, X. Ye, X. Liu, and E. D. Louis, “Genetic analysis of FMR1 repeat expansion in essential tremor,” Neurosci. Lett., 593, 114–117 (2015), https://doi.org/10.1016/j.neulet.2015.03.027.
P. J. Morrison and J. Benito-León, “Neurologic features in intermediate allele carriers of Huntington disease,” Neurology, 87, No. 6, 556–557 (2016), https://doi.org/10.1212/WNL.0000000000002958.
M. van Hagen, D. G. E. Piebes, W. C. de Leeuw, et al., “The dynamics of early-state transcriptional changes and aggregate formation in a Huntington’s disease cell model,” BMC Genomics, 18, No. 1, 373 (2017), https://doi.org/10.1186/s12864-017-3745-z.
C. Marques Sousa and S. Humbert, “Huntingtin: here, there, everywhere!,” J. Huntingtons Dis., 2, No. 4, 395–403 (2013), https://doi.org/10.3233/JHD-130082.
D. Rigamonti, J. H. Bauer, C. De-Fraja, et al., “Wild-type huntingtin protects from apoptosis upstream of caspase-3,” J. Neurosci., 20, No. 10, 3705–3713 (2000), https://doi.org/10.1523/JNEUROSCI.20-10-03705.2000.
M. H. Schaefer, E. E. Wanker, and M. A. Andrade-Navarro, “Evolution and function of CAG/polyglutamine repeats in protein–protein interaction networks,” Nucleic Acids Res., 40, No. 10, 4273–4287 (2012), https://doi.org/10.1093/nar/gks011.
F. Sampedro, S. Martinez-Horta, J. Pérez-Pérez, et al., “Interaction between sex and neurofilament light chain on brain structure and clinical severity in Huntington’s disease,” Ann. Clin. Transl. Neurol., 8, No. 12, 2309–2313 (2021), https://doi.org/10.1002/acn3.51460.
C. Riccardi, F. Napolitano, D. Montesarchio, et al., “Nanoparticleguided brain drug delivery: Expanding the therapeutic approach to neurodegenerative diseases,” Pharmaceutics, 13, No. 11, 1897 (2021), https://doi.org/10.3390/pharmaceutics13111897.
D. Savitt and J. Jankovic, “Clinical phenotype in carriers of intermediate alleles in the huntingtin gene,” J. Neurol. Sci., 402, 57–61 (2019), https://doi.org/10.1016/j.jns.2019.05.010.
C. M. Testa and J. Jankovic, “Huntington disease: A quarter century of progress since the gene discovery,” J. Neurol. Sci., 396, 52–68 (2019), https://doi.org/10.1016/j.jns.2018.09.022.
N. R. Downing, S. Lourens, I. De Soriano, et al. “Phenotype characterization of HD intermediate alleles in PREDICT-HD,” J. Huntingtons Dis., 5, No. 4, 357–368 (2016), https://doi.org/10.3233/JHD-160185.
Huntington Study Group PHAROS Investigators, K. M. Biglan, I. Shoulson, et al., “Clinical-genetic associations in the Prospective Huntington at Risk Observational Study (PHAROS, implications for clinical trials,” JAMA Neurol., 73, No. 1, 102-10 (2016), PMID: 26569098, https://doi.org/10.1001/jamaneurol.2015.2736.
A. D. Ha, C. A. Beck, and J. Jankovic, “Intermediate CAG repeats in Huntington’s disease: Analysis of COHORT,” Tremor Other Hyperkinet. Mov. (NY), 2, tre-02-64-287-4 (2012), https://doi.org/10.7916/D8FF3R2P.
M. Leija-Salazar, C. Piette, and C. Proukakis, “Review: Somatic mutations in neurodegeneration,” Neuropathol. Appl. Neurobiol., 44, No. 3, 267–285 (2018), https://doi.org/10.1111/nan.12465.
D. Falush, E. W. Almqvist, R. R. Brinkmann, et al., “Measurement of mutational fl ow implies both a high new-mutation rate for Huntington disease and substantial underascertainment of late-onset cases,” Am. J. Hum. Genet., 68, No. 2, 373–385 (2001), https://doi.org/10.1086/318193.
C. Kay, J. A. Collins, G. E. B. Wright, et al., “The molecular epidemiology of Huntington disease is related to intermediate allele frequency and haplotype in the general population,” Am J. Med. Genet. B Neuropsychiatr. Genet., 177, No. 3, 346–357 (2018), https://doi.org/10.1002/ajmg.b.32618.
A. Semaka, L. G. Balneaves, and M. R. Hayden, “’Grasping the grey’: patient understanding and interpretation of an intermediate allele predictive test result for Huntington disease,” J. Genet. Couns., 22, No. 2, 200–217 (2013), https://doi.org/10.1007/s10897-012-9533-7.
A. E. Hendricks, J. C. Latourelle, K. L. Lunetta, et al., “Estimating the probability of de novo HD cases from transmissions of expanded penetrant CAG alleles in the Huntington disease gene from male carriers of high normal alleles (27–35 CAG),” Am. J. Med. Genet. A, 149A, No. 7, 1375–1381 (2009), https://doi.org/10.1002/ajmg.a.32901.
A. Semaka, C. Kay, C. Doty, et al., “CAG size-specific risk estimates for intermediate allele repeat instability in Huntington disease,” J. Med. Genet., 50, No. 10, 696–703 (2013), https://doi.org/10.1136/jmedgenet-2013-101796.
H. Telenius, B. Kremer, Y. P. Goldberg, et al., “Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm,” Nat. Genet., 6, No. 4, 409–414 (1994), https://doi.org/10.1038/ng0494-409.
A. Semaka, C. Kay, R. D. Belfroid, et al., “A new mutation for Huntington disease following maternal transmission of an intermediate allele,” Eur. J. Med. Genet., 58, No. 1, 28–30 (2015), https://doi.org/10.1016/j.ejmg.2014.11.005.
R. MacLeod, A. Tibben, M. Frontali, et al., “Editorial Committee and Working Group ‘Genetic Testing Counselling’ of the European Huntington Disease Network. Recommendations for the predictive genetic test in Huntington’s disease,” Clin. Genet., 83, No. 3, 221–231 (2013), https://doi.org/10.1111/j.1399-0004.2012.01900.x.
A. Semaka and M. R. Hayden, “Evidence-based genetic counselling implications for Huntington disease intermediate allele predictive test results,” Clin. Genet., 85, No. 4, 303–311 (2014), https://doi.org/10.1111/cge.12324.
F. Squitieri and J. Jankovic, “Huntington’s disease: how intermediate are intermediate repeat lengths?” Mov. Disord., 27, No. 14, 1714–1717 (2012), https://doi.org/10.1002/mds.25172.
L. Bean, P. Bayrak-Toydemir, and the ACMG Laboratory Quality Assurance Committee, “Addendum: American College of Medical Genetics and Genomics Standards and Guidelines for Clinical Genetics Laboratories, 2014 edition: technical standards and guidelines for Huntington disease,” Genet. Med., 23, No. 12, 2461 (2021), https://doi.org/10.1038/s41436-020-0893-3.
S. N. Illarioshkin, S. A. Klyushnikov, and Yu. A. Seliverstov, Huntington’s Disease, Atmosfera Press, Moscow (2018), https://doi.org/10.12731/978-5-902123-69-9.
J. Jankovic and T. Ashizawa, “Tourettism associated with Huntington’s disease,” Mov. Disord., 10, No. 1, 103–105 (1995), https://doi.org/10.1002/mds.870100116.
A. Ng and E. Tan, “Intermediate C9orf72 alleles in neurological disorders: does size really matter?” J. Med. Genet., 54, 591–597 (2017).
S. Migliore, J. Jankovic, and F. Squitieri, “Genetic counseling in Huntington’s disease: Potential new challenges on horizon?” Front. Neurol., 10, 453 (2019), https://doi.org/10.3389/fneur.2019.00453.
G. M. McKhann, D. S. Knopman, H. Chertkow, et al., “The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,” Alzheimers Dement., 7, No. 3, 263–269 (2011), https://doi.org/10.1016/j.jalz.2011.03.005.
R. B. Postuma, D. Berg, M. Stern, et al., “MDS clinical diagnostic criteria for Parkinson’s disease,” Mov. Disord., 30, No. 12, 1591–1601 (2015), https://doi.org/10.1002/mds.26424.
A. Semaka, S. Warby, B. R. Leavitt, and M. R. Hayden, “Re: Autopsy-proven Huntington’s disease with 29 trinucleotide repeats,” Mov. Disord., 23, No. 12, 1794–1795, author reply 1793 (2008), https://doi.org/10.1002/mds.21820.
M. Y. Davis, C. D. Keene, S. Jayadev, and T. Bird, “The co-occurrence of Alzheimer’s disease and Huntington’s disease: a neuropathological study of 15 elderly Huntington’s disease subjects,” J. Huntingtons Dis., 3, No. 2, 209–217 (2014), https://doi.org/10.3233/JHD-140111.
K. Bürger, R. Mergner, V. Arbusow, et al., ”Chorea Huntington mit später Manifestation als Differenzialdiagnose der Alzheimer-Krankheit,” Nervenarzt, 73, No. 9, 870–873 (2002), https://doi.org/10.1007/s00115-002-1361-9.
E. Mulroy, A. Latorre, E. Menozzi, et al., “Huntington disease like 2 (HDL-2) with parkinsonism and abnormal DAT-SPECT – A novel observation,” Parkinsonism Relat. Disord., 71, 46–48 (2020), https://doi.org/10.1016/j.parkreldis.2020.01.008.
Y. M. Sun, Y. B. Zhang, and Z. Y. Wu, “Huntington’s disease: Relationship between phenotype and genotype,” Mol. Neurobiol., 54, No. 1, 342–348 (2017), https://doi.org/10.1007/s12035-015-9662-8.
J. K. Lee, A. Conrad, E. Epping, et al., “Effect of trinucleotide repeats in the Huntington’s gene on intelligence,” EBioMedicine, 31, 47–53 (2018), https://doi.org/10.1016/j.ebiom.2018.03.031.
S. L. Gardiner, M. J. van Belzen, M. W. Boogaard, et al., “Huntingtin gene repeat size variations affect risk of lifetime depression,” Transl. Psychiatry, 7, No. 12, 1277 (2017), PMCID: 29225330, PMC5802693, https://doi.org/10.1038/s41398-017-0042-1.
S. L. Gardiner, M. J. van Belzen, M. W. Boogaard, et al., “Large normal-range TBP and ATXN7 CAG repeat lengths are associated with increased lifetime risk of depression,” Transl. Psychiatry, 7, No. 6, e1143 (2017), https://doi.org/10.1038/tp.2017.116.
R. R. Bogdanov, S. Yu. Borisova, S. V. Kotov, and O. O. Zavarzina, “The personality profile of patients with early manifestations of Parkinson’s disease,” Alman. Klin. Med., 44, No. 3, 329–335 (2016), https://doi.org/10.18786/2072-0505-2016-44-3-329-335.
R. Dewan, R. Chia, J. Ding, et al., “Pathogenic Huntingtin repeat expansions in patients with frontotemporal dementia and amyotrophic lateral sclerosis,” Neuron, 109, No. 3, 448–460.e4 (2021), https://doi.org/10.1016/j.neuron.2020.11.005.
F. Squitieri, M. Esmaeilzadeh, A. Ciarmiello, and J. Jankovic, “Caudate glucose hypometabolism in a subject carrying an unstable allele of intermediate CAG(33) repeat length in the Huntington’s disease gene,” Mov. Disord., 26, No. 5, 925–927 (2011), https://doi.org/10.1002/mds.23623.
J. L. Groen, R. M. de Bie, E. M. Foncke, et al., “Late-onset Huntington disease with intermediate CAG repeats: true or false?” J. Neurol. Neurosurg. Psychiatry, 81, No. 2, 228–230 (2010), https://doi.org/10.1136/jnnp.2008.170902.
M. A. Nikitina, E. Yu. Bragina, and D. E. Gomboeva, et al., “Atypical course of Parkinson’s disease with clinical manifestations of Huntington’s disease in a patient with an allele of 27 CAG repeats in the HTT gene,” Byull. Sibirsk. Med., M. A. 19, No. 4, 235–240 (2020), https://doi.org/10.20538/1682-0363-2020-4-235-240.
E. Cubo, M. A. Ramos-Arroyo, S. Martinez-Horta, et al., European HD Network, “Clinical manifestations of intermediate allele carriers in Huntington disease,” Neurology, 87, No. 6, 571–578 (2016), https://doi.org/10.1212/WNL.0000000000002944.
A. D. Ha and J. Jankovic, “Exploring the correlates of intermediate CAG repeats in Huntington disease,” Postgrad. Med., 123, No. 5, 116–121 (2011), https://doi.org/10.3810/pgm.2011.09.2466.
J. Sequeiros, E. M. Ramos, J. Cerqueira, et al., “Large normal and reduced penetrance alleles in Huntington disease: instability in families and frequency at the laboratory, at the clinic and in the population,” Clin. Genet., 78, No. 4, 381–387 (2010), https://doi.org/10.1111/j.1399-0004.2010.01388.x.
T. B. Stoker, S. T. Holden, and R. A. Barker, “Late-onset Huntington’s disease associated with CAG repeat lengths of 30 and 31,” J. Neurol., 268, No. 10, 3916–3919 (2021), https://doi.org/10.1007/s00415-021-10633-3.
S. D. Jevtic and J. P. Provias, “Case report and literature review of Huntington disease with intermediate CAG expansion,” BMJ Neurol. Open, 2, No. 1, e000027 (2020), https://doi.org/10.1136/bmjno-2019-000027.
S. R. Chintalaphani, S. S. Pineda, I. W. Deveson, and K. R. Kumar, “An update on the neurological short tandem repeat expansion disorders and the emergence of long-read sequencing diagnostics,” Acta Neuropathol. Comm., 9, No. 1, 45–49 (2021), https://doi.org/10.1186/s40478-021-01201-x.
A. S. L. Ng and E. Tan, “Intermediate C9orf72 alleles in neurological disorders: does size really matter?” J. Med. Genet., 54, 591–597 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 122, No. 7, Iss. 1, pp. 42–50, July, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikitina, M.A., Bragina, E.Y., Nazarenko, M.S. et al. The Role of Alleles with Intermediate Numbers of Trinucleotide Repeats in Parkinson’s Disease and Other Neurodegenerative Diseases. Neurosci Behav Physi 53, 193–201 (2023). https://doi.org/10.1007/s11055-023-01408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01408-6