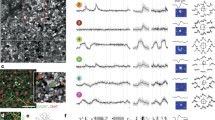
The visual conducting pathways of higher mammals consist of three main channels: X, Y, and W in carnivores and the magno-, parvo-, and koniocellular pathways in primates; neurons in these channels differ in terms of body area, extent of dendrite branching, and axon thickness, which determine the characteristic properties of their receptive fields. The structural-functional organization of the first two channels – X/parvo and Y/magno – have been analyzed in many experimental and theoretical studies, and have been addressed in informative reviews and monographs; the characteristics of the organization of the third (W/konio) conducting channel have long attracted much less attention, and its large-scale study has only started relatively recently. The aim of the present work was to undertake a comparative analysis of existing data on the structure and functions of the third conducting channel in a number of mammals of the orders Carnivora and Primates.
Similar content being viewed by others
References
Abramson, B. P. and Chalupa, L. M., “The laminar distribution of cortical connections with the tecto- and cortico-recipient zones in the cat’s lateral posterior nucleus,” Neuroscience, 15, 81–95 (1985).
Adams, M. M., Hof, P. R., Gattass, R., et al., “Visual cortical projections and chemoarchitecture of macaque monkey pulvinar,” J. Comp. Neurol., 419, 377–393 (2000).
Ajina, S., Rees, G., Kennard, C., and Bridge, H., “Abnormal contrast responses in the extrastriate cortex of blindsight patients,” J. Neurosci., 35, 8201–8213 (2015).
Anderson, J. C., da Costa, N. M., and Martin, K. A., “The W cell pathway to cat primary visual cortex,” J. Comp. Neurol., 516, 20–35 (2009).
Baldwin, M. K. L and Krubitzer, L., “Architectonic characteristics of the visual thalamus and superior colliculus in titi monkeys,” J. Comp. Neurol., 526, 1760–1776 (2018).
Ballas, I., Hoffmann, K. P., and Wagner, H. J., “Retinal projection to the nucleus of the optic tract in the cat as revealed by retrograde transport of horseradish peroxidase,” Neurosci. Lett., 26, 197–202 (1981).
Beck, P. D. and Kaas, J. H., “Thalamic connections of the dorsomedial visual area in primates,” J. Comp. Neurol., 396, 381–398 (1998).
Behan, M., Steinhacker, K., Jeffrey-Borger, S., and Meredith, M. A., “Chemoarchitecture of GABAergic neurons in the ferret superior colliculus,” J. Comp. Neurol., 452, 334–359 (2002).
Benevento, L. A. and Yoshida, K., “The afferent and efferent organization of the lateral geniculo-prestriate pathways in the macaque monkey,” J. Comp. Neurol., 203, 455–474 (1981).
Berson, D. M., “Retinal W-cell input to the upper superficial gray layer of the cat’s superior colliculus: a conduction-velocity analysis,” J. Neurophysiol., 58, 1035–1051 (1987).
Berson, D. M., Isayama, T., and Pu, M., “The Eta ganglion cell type of cat retina,” J. Comp. Neurol., 408, 204–219 (1999).
Berson, D. M., Pu, M., and Famiglietti, E. V., “The zeta cell: a new ganglion cell type in cat retina,” J. Comp. Neurol., 399, 269–288 (1998).
Boycott, B. B. and Wässle, H., “The morphological types of ganglion cells of the domestic cat’s retina,” J. Physiol., 240, 397–419 (1974).
Boyd, J. D. and Casagrande, V. A., “Relationships between cytochrome oxidase (CO) blobs in primate primary visual cortex (V1) and the distribution of neurons projecting to the middle temporal area (MT),” J. Comp. Neurol., 409, 573–591 (1999).
Boyd, J. D. and Matsubara, J. A., “Projections from V1 to lateral suprasylvian cortex: an efferent pathway in the cat’s visual cortex that originates preferentially from CO blob columns,” Vis. Neurosci., 16, 849–860 (1999).
Buzás, P., Kóbor, P., Petykó, Z., et al., “Receptive field properties of color opponent neurons in the cat lateral geniculate nucleus,” J. Neurosci., 33, 1451–1461 (2013).
Callaway, E. M., “Structure and function of parallel pathways in the primate early visual system,” J. Physiol., 566, 13–9 (2005).
Casagrande, V. A., “A third parallel visual pathway to primate area V1,” Trends Neurosci., 17, 305–310 (1994).
Casagrande, V. A., Khaytin, I., and Boyd, J., “The evolution of parallel visual pathways in the brains of primates,” in: Evolution of the Nervous System, Preuss, T. M. and Kaas J (eds.) (2007a), Vol. 4, p. 87–108.
Casagrande, V. A., Yazar, F., Jones, K. D., and Ding, Y., “The morphology of the koniocellular axon pathway in the macaque monkey,” Cereb. Cortex, 17, 2334–2345 (2007b).
Chen, B., Hu, X. J., and Pourcho, R. G., “Morphological diversity in terminals of W-type retinal ganglion cells at projection sites in cat brain,” Vis. Neurosci., 13, 449–460 (1996).
Cheong, S. K., Tailby, C., Martin, P. R., et al., “Slow intrinsic rhythm in the koniocellular visual pathway,” Proc. Natl. Acad. Sci. USA, 108, 14659–14663 (2011).
Cleland, B. G. and Levick, W. R., “Brisk and sluggish concentrically organized ganglion cells in the cat’s retina,” J. Physiol., 240, 421–456 (1974a).
Cleland, B. G. and Levick, W. R., “Properties of rarely encountered types of ganglion cells in the cat’s retina and an overall classifi cation,” J. Physiol., 240, 457–492 (1974b).
Condo, G. J. and Casagrande, V. A., “Organization of cytochrome oxidase staining in the visual cortex of nocturnal primates (Galago crassicaudatus and Galago senegalensis): I. Adult patterns,” J. Comp. Neurol., 293, 632–645 (1990).
Conley, M., Fitzpatrick, D., and Diamond, I. T., “The laminar organization of the lateral geniculate body and the striate cortex in the tree shrew (Tupaia glis),” J. Neurosci., 4, 171–197 (1984).
Costa, M. S. and Britto, L. R., “Calbindin immunoreactivity delineates the circadian visual centers of the brain of the common marmoset (Callithrix jacchus),” Brain Res. Bull., 43, 369–373 (1997).
Costa, M. S. M. O., Moreira, L. F., Alones, V., et al., “Characterization of the circadian system in a Brazilian species of monkey (Callithrix jacchus): immunohistochemical analysis and retinal projections,” Biol. Rhythm Res., 29, 510–520 (1998).
Cottaris, N. P. and De Valois, R. L., “Temporal dynamics of chromatic tuning in macaque primary visual cortex,” Nature, 395, 896–900 (1998).
Cowey, A., Johnson, H., and Stoerig, P., “The retinal projection to the pregeniculate nucleus in normal and destriate monkeys,” Eur. J. Neurosci., 13, 279–290 (2001).
Cowey, A., Stoerig, P., and Bannister, M., “Retinal ganglion cells labelled from the pulvinar nucleus in macaque monkeys,” Neuroscience, 61, 691–705 (1994).
Crook, J. D., Peterson, B. B., Packer, O. S., et al., “The smooth monostratified ganglion cell: evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey,” J. Neurosci., 28, 12,654–12,671 (2008).
Cropper, S. J. and Derrington, A. M., “Rapid colour-specifi c detection of motion in human vision,” Nature, 379, 72–74 (1996).
Cui He and Malpeli, J. G., “Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets,” J. Neurophysiol., 89, 3128–3142 (2003).
Cusick, C. G., Scripter, J. L., Darensbourg, J. G., and Weber, J. T., “Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr,” J. Comp. Neurol., 336, 1–30 (1993).
Dacey, D. M. and Lee, B. B., “The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type,” Nature, 367, 731–735 (1994).
Dacey, D. M. and Packer, O. S., “Colour coding in the primate retina: diverse cell types and cone-specifi c circuitry,” Curr. Opin. Neurobiol., 13, 421–427 (2003).
Dacey, D. M., “Morphology of a small-field bistratified ganglion cell type in the macaque and human retina,” Vis. Neurosci., 10, 1081–1098 (1993).
Dacey, D. M., Peterson, B. B., Robinson, F. R., and Gamlin, P. D., “Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types,” Neuron, 37, 15–27 (2003).
Danckert, J. and Rossetti, Y., “Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions?” Neurosci. Biobehav. Rev., 29, 1035–1046 (2005).
Daw, N. W. and Pearlman, A. L., “Cat colour vision: evidence for more than one cone process,” J. Physiol., 211, 125–137 (1970).
de Monasterio, F. M. and Gouras, P., “Functional properties of ganglion cells of the rhesus monkey retina,” J. Physiol., 251, 167–195 (1975).
DeBruyn, E. J., Casagrande, V. A., Beck, P. D., and Bonds, A. B., “Visual resolution and sensitivity of single cells in the primary visual cortex (V1) of a nocturnal primate (bush baby, correlations with cortical layers and cytochrome oxidase patterns,” J. Neurophysiol., 69, 3–18 (1993).
Denny-Brown, D. and Chambers, R. A., “Physiological aspects of visual perception. I. Functional aspects of visual cortex,” Arch. Neurol., 33, 219–227 (1976).
Di Stefano, M., Morrone, M. C., and Burr, D. C., “Visual acuity of neurones in the cat lateral suprasylvian cortex,” Brain Res., 331, 382– 385 (1985).
Diamond, I. T., Conley, M., Itoh, K., and Fitzpatrick, D., “Laminar organization of geniculocortical projections in Galago senegalensis and Aotus trivirgatus,” J. Comp. Neurol., 242, 584–610 (1985).
Diamond, I. T., Fitzpatrick, D., and Conley, M., “A projection from the parabigeminal nucleus to the pulvinar nucleus in Galago,” J. Comp. Neurol., 316, 375–382 (1992).
Diamond, I. T., Fitzpatrick, D., and Schmechel, D., “Calcium binding proteins distinguish large and small cells of the ventral posterior and lateral geniculate nuclei of the prosimian galago and the tree shrew (Tupaia belangeri),” Proc. Natl. Acad. Sci. USA, 90, 1425–1429 (1993).
Ding, Y. and Casagrande, V. A., “The distribution and morphology of LGN K pathway axons within the layers and CO blobs of owl monkey V1,” Vis. Neurosci., 14, 691–704 (1997).
Do, M. T. H. and Yau, K.-W., “Intrinsically photosensitive retinal ganglion cells,” Physiol. Rev., 90, 1547–1581 (2010).
Dreher, B., Leventhal, A. G., and Hale, P. T., “Geniculate input to cat visual cortex: a comparison of area 19 with areas 17 and 18,” J. Neurophysiol., 44, 804–826 (1980).
Eiber, C. D., Rahman, A. S., Pietersen, A. N. J., et al., “Receptive field properties of koniocellular On/Off neurons in the lateral geniculate nucleus of marmoset monkeys,” J. Neurosci., 38, 10384–10398 (2018).
Famiglietti, E. V., “Dendritic co-stratifi cation of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina,” J. Comp. Neurol., 324, 322–335 (1992).
Famiglietti, E. V., “Starburst amacrine cells in cat retina are associated with bistratified, presumed directionally selective, ganglion cells,” Brain Res., 413, 404–408 (1987).
Farmer, S. G. and Rodieck, R. W., “Ganglion cells of the cat accessory optic system: morphology and retinal topography,” J. Comp. Neurol., 205, 190–198 (1982).
Feig, S. and Harting, J. K., “Ultrastructural studies of the primate lateral geniculate nucleus: morphology and spatial relationships of axon terminals arising from the retina, visual cortex (area 17), superior colliculus, parabigeminal nucleus, and pretectum of Galago crassicaudatus,” J. Comp. Neurol., 343, 17–34 (1994).
Felch, D. L. and Van Hooser, S. D., “Molecular compartmentalization of lateral geniculate nucleus in the gray squirrel (Sciurus carolinensis),” Front. Neuroanat., 6, 1–12 (2012).
Fitzpatrick, D., Itoh, K., and Diamond, I. T., “The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus),” J. Neurosci., 3, 673–702 (1983).
Fukada, Y., “Receptive field organization of cat optic nerve fi bers with special reference to conduction velocity,” Vision Res., 11, 209–226 (1971).
Fukuda, Y. and Stone, J., “Retinal distribution and central projections of Y, X, and W-cells of the cat’s retina,” J. Neurophysiol., 37, 749–772 (1974).
Fukuda, Y., Hsiao, C. F., Watanabe, M., and Ito, H., “Morphological correlates of physiologically identified Y-, X-, and W-cells in cat retina,” J. Neurophysiol., 52, 999–1013 (1984).
Ghodrati, M., Khaligh-Razavi, S. M., and Lehky, S. R., “Towards building a more complex view of the lateral geniculate nucleus: Recent advances in understanding its role,” Prog. Neurobiol., 156, 214–255 (2017).
Ghosh, K. K. and Grünert, U., “Synaptic input to small bistratified (blue- ON) ganglion cells in the retina of a new world monkey, the marmoset Callithrix jacchus,” J. Comp. Neurol., 413, 417–428 (1999).
Giolli, R. A., Blanks, R. H., and Lui, F., “The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function,” Prog. Brain Res., 151, 407–440 (2006).
Gollisch, T. and Meister, M., “Eye smarter than scientists believed: neural computations in circuits of the retina,” Neuron, 65, 150–164 (2010).
Goodchild, A. K. and Martin, P. R., “The distribution of calcium-binding proteins in the lateral geniculate nucleus and visual cortex of a New World monkey, the marmoset, Callithrix jacchus,” Vis. Neurosci., 15, 625–642 (1998).
Gray, D., Gutierrez, C., and Cusick, C. G., “Neurochemical organization of inferior pulvinar complex in squirrel monkeys and macaques revealed by acetylcholinesterase histochemistry, calbindin and Cat- 301 immunostaining, and Wisteria fl oribunda agglutinin binding,” J. Comp. Neurol., 409, 452–468 (1999).
Graybiel, A. M., “A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superfi cial collicular layers,” Brain Res., 145, 365–374 (1978).
Guenther, E. and Zrenner, E., “The spectral sensitivity of dark- and light-adapted cat retinal ganglion cells,” J. Neurosci., 13, 1543–1550 (1993).
Harting, J. K., Casagrande, V. A., and Weber, J. T., “The projection of the primate superior colliculus upon the dorsal lateral geniculate nucleus: autoradiographic demonstration of interlaminar distribution of tectogeniculate axons,” Brain Res., 150, 593–599 (1978).
Harting, J. K., Hashikawa, T., and Van Lieshout, D., “Laminar distribution of tectal, parabigeminal and pretectal inputs to the primate dorsal lateral geniculate nucleus: connectional studies in Galago crassicaudatus,” Brain Res., 366, 358–363 (1986).
Harting, J. K., Huerta, M. F., Hashikawa, T., and van Lieshout, D. P., “Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species,” J. Comp. Neurol., 304, 275–306 (1991).
Hashikawa, T., Van Lieshout, D., and Harting, J. K., “Projections from the parabigeminal nucleus to the dorsal lateral geniculate nucleus in the tree shrew Tupaia glis,” J. Comp. Neurol., 246, 382–394 (1986).
Hassler, R., “Comparative anatomy of the central visual systems in dayand night-active primates,” in: Evolution of the Forebrain, Hassler, R. and Stephan H (eds), Springer, Boston, MA (1966).
Hendry, S. H. and Yoshioka, T., “A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus,” Science, 264, 575–577 (1994).
Hendry, S. H. C. and Reid, R. C., “The koniocellular pathway in primate vision,” Annu. Rev. Neurosci., 23, 127–153 (2000).
Hendry, S. H., Fuchs, J., deBlas, A. L., and Jones, E. G., “Distribution and plasticity of immunocytochemically localized GABAA receptors in adult monkey visual cortex,” J. Neurosci., 10, 2438–2450 (1990).
Hernández-González, A., Cavada, C., and Reinoso-Suárez, F., “The lateral geniculate nucleus projects to the inferior temporal cortex in the macaque monkey,” Neuroreport, 5, 2693–2696 (1994).
Hess, D. T. and Edwards, M. A., “Anatomical demonstration of ocular segregation in the retinogeniculocortical pathway of the New World capuchin monkey (Cebus apella),” J. Comp. Neurol., 264, 409–420 (1987).
Hoffmann, K.-P., “Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive field properties,” J. Neurophysiol., 36, 409–424 (1973).
Holländer, H. and Vanegas, H., and “The projection from the lateral geniculate nucleus onto the visual cortex in the cat. A quantitative study with horseradish-peroxidase,” J. Comp. Neurol., 173, 519–536 (1977).
Horton, J. C. and Hocking, D. R., “Monocular core zones and binocular border strips in primate striate cortex revealed by the contrasting effects of enucleation, eyelid suture, and retinal laser lesions on cytochrome oxidase activity,” J. Neurosci., 18, 5433–5455 (1998).
Horton, J. C., “Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex,” Philos. Trans. R. Soc. Lond. B Biol. Sci., 304, 199–253 (1984).
Hubel, D. H., “Blobs and color vision,” Cell Biophys., 9, 91–102 (1986).
Hughes, C. P. and Ater, S. B., “Receptive field properties in the ventral lateral geniculate nucleus of the cat,” Brain Res., 132, 163–166 (1977).
Huo, B.-X., Zeater, N., Lin, M. K., et al., “Relation of koniocellular layers of dorsal lateral geniculate to inferior pulvinar nuclei in common marmosets,” Eur. J. Neurosci., 50, 4004–4017 (2019).
Irvin, G. E., Norton, T. T., Sesma, M. A., and Casagrande, V. A., “W-like response properties of interlaminar zone cells in the lateral geniculate nucleus of a primate (Galago crassicaudatus),” Brain Res., 362, 254–270 (1986).
Isayama, T., Berson, D. M., and Pu, M., “Theta ganglion cell type of cat retina,” J. Comp. Neurol., 417, 32–48 (2000).
Isayama, T., O’Brien, B. J., Ugalde, I., et al., “Morphology of retinal ganglion cells in the ferret (Mustela putorius furo),” J. Comp. Neurol., 517, 459–480 (2009).
Itaya, S. K. and Van Hoesen, G. W., “Retinal projections to the inferior and medial pulvinar nuclei in the Old-World monkey,” Brain Res., 269, 223–230 (1983).
Itoh, K., Conley, M., and Diamond, I. T., “Different distribution of large and small retinal ganglion cells in the cat after HRP injections of single layers of the lateral geniculate body and the superior colliculus,” Brain Res., 207, 147–152 (1981).
Itoh, K., Conley, M., and Diamond, I. T., “Retinal ganglion cell projections to individual layers of the lateral geniculate body in Galago crassicaudatus,” J. Comp. Neurol., 205, 282–290 (1982).
Jacobs, G. H., Neitz, M., and Neitz, J., “Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate,” Proc. Biol. Sci., 263, 705–710 (1996).
Johnson, J. K. and Casagrande, V. A., “Distribution of calcium-binding proteins within the parallel visual pathways of a primate (Galago crassicaudatus),” J. Comp. Neurol., 356, 238–260 (1995).
Jones, E. G., “The thalamic matrix and thalamocortical synchrony,” Trends Neurosci., 24, 595–601 (2001).
Kaas, J. H., “Blindsight: post-natal potential of a transient pulvinar pathway,” Curr. Biol., 25, R155–157 (2015).
Kaas, J. H., Harting, J. K., and Guillery, R. W., “Representation of the complete retina in the contralateral superior colliculus of some mammals,” Brain Res., 65, 343–346 (1974).
Kaplan, E., The M, K, and P Streams in the Primate Visual System: What Do They Do for Vision?, Masland, R. and Albright, T (eds.), Elsevier, UK (2008).
Kawano, J., “Cortical projections of the parvocellular laminae C of the dorsal lateral geniculate nucleus in the cat: an anterograde wheat germ agglutinin conjugated to horseradish peroxidase study,” J. Comp. Neurol., 392, 439–457 (1998).
Klein, C., Evrard, H. C., Shapcott, K. A., et al., “Cell-targeted optogenetics and electrical microstimulation reveal the primate koniocellular projection to supragranular visual cortex,” Neuron, 90, 143–151 (2016).
Kolb, H., Nelson, R., and Mariani, A., “Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study,” Vision Res., 21, 1081– 1114 (1981).
Koontz, M. A., Rodieck, R. W., and Farmer, S. G., “The retinal projection to the cat pretectum,” J. Comp. Neurol., 236, 42–59 (1985).
Lachica, E. A. and Casagrande, V. A., “Direct W-like geniculate projections to the cytochrome oxidase (CO) blobs in primate visual cortex: axon morphology,” J. Comp. Neurol., 319, 141–158 (1992).
Lachica, E. A. and Casagrande, V. A., “The morphology of collicular and retinal axons ending on small relay (W-like) cells of the primate lateral geniculate nucleus,” Vis. Neurosci., 10, 403–418 (1993).
Lennie, P., “Parallel visual pathways: a review,” Vision Res., 20, 561–594 (1980).
Leopold, D. A., “Primary visual cortex: awareness and blindsight,” Annu. Rev. Neurosci., 35, 91–109 (2012).
LeVay, S. and Gilbert, C. D., “Laminar patterns of geniculocortical projection in the cat,” Brain Res., 113, 1–19 (1976).
Leventhal, A. G., “Evidence that the different classes of relay cells of the cat’s lateral geniculate nucleus terminate in different layers of the striate cortex,” Exp. Brain Res., 37, 349–372 (1979).
Leventhal, A. G., Keens, J., and Törk, I., “The afferent ganglion cells and cortical projections of the retinal recipient zone (RRZ) of the cat’s pulvinar complex,” J. Comp. Neurol., 194, 535–554 (1980).
Leventhal, A. G., Rodieck, R. W., and Dreher, B., “Central projections of cat retinal ganglion cells,” J. Comp. Neurol., 237, 216–226 (1985).
Lin, C. S. and Kaas, J. H., “Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex,” Neuroscience, 5, 2219–2228 (1980).
Livingston, C. A. and Fedder, S. R., “Visual-ocular motor activity in the macaque pregeniculate complex,” J. Neurophysiol., 90, 226–244 (2003).
Livingstone, M. and Hubel, D., “Segregation of form, color, movement, and depth: anatomy, physiology, and perception,” Science, 240, 740–749 (1988).
Lysakowski, A., Standage, G. P., and Benevento, L. A., “An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study,” Exp. Brain Res., 69, 651–661 (1988).
Ma, R., Cui, H., Lee, S.-H., et al., “Predictive encoding of moving target trajectory by neurons in the parabigeminal nucleus,” J. Neurophysiol., 109, 2029–2043 (2013).
Marshak, D. W. and Mills, S. L., “Short-wavelength cone-opponent retinal ganglion cells in mammals,” Vis. Neurosci., 31, 165–175 (2014).
Martin, P. R. and Grünert, U., “Analysis of the short wavelength-sensitive (‘blue’) cone mosaic in the primate retina: comparison of New World and Old World monkeys,” J. Comp. Neurol., 406, 1–14 (1999).
Martin, P. R. and Lee, B. B., “Distribution and specifi city of S-cone (‘blue cone’) signals in subcortical visual pathways,” Vis. Neurosci., 31, 177–187 (2014).
Martin, P. R. and Solomon, S. G., “The koniocellular whiteboard,” J. Comp. Neurol., 527, No. 3, 505–507 (2018).
Martin, P. R., White, A. J., Goodchild, A. K., et al., “Evidence that blue-on cells are part of the third geniculocortical pathway in primates,” Eur. J. Neurosci., 9, 1536–1541 (1997).
Mason, R., “Differential responsiveness of cells in the visual zones of the cat’s LP-pulvinar complex to visual stimuli,” Exp. Brain Res., 43, 25–33 (1981).
Mass, A. M. and Supin, A. Y., “Ganglion cells density and retinal resolution in the sea otter, Enhydra lutris,” Brain Behav. Evol., 55, 111–119 (2000).
Merigan, W. H. and Maunsell, J. H., “How parallel are the primate visual pathways?” Annu. Rev. Neurosci., 16, 369–402 (1993).
Merkulyeva, N. S., “Conducting channels in the visual system. Basis of classification,” Zh. Vyssh. Nerv. Deyat., 69, 541–549 (2019).
Merkulyeva, N. S., Mikhalkin, A. A., and Nikitina, N. I., “Postnatal development of the posterolateral nuclear complex of the dorsal thalamus,” Integrat. Fiziol., 1, 242–248 (2020).
Merkulyeva, N. S., Mikhalkin, A. A., and Veshchitskii, A. A., “Characteristics of the distribution of acetylcholinesterase in the posterolateral nucleus of the thalamus in cats,” Morfologiya, 148, 46–48 (2015).
Mitzdorf, U. and Singer, W., “Laminar segregation of afferents to lateral geniculate nucleus of the cat: an analysis of current source density,” J. Neurophysiol., 40, 1227–1244 (1977).
Mize, R. R. and Horner, L. H., “Retinal synapses of the cat medial interlaminar nucleus and ventral lateral geniculate nucleus differ in size and synaptic organization,” J. Comp. Neurol., 224, 579–590 (1984).
Mize, R. R., “Calbindin 28kD and parvalbumin immunoreactive neurons receive different patterns of synaptic input in the cat superior colliculus,” Brain Res., 843, 25–35 (1999).
Mize, R. R., “Neurochemical microcircuitry underlying visual and oculomotor function in the cat superior colliculus,” Prog. Brain Res., 112, 35–55 (1996).
Mize, R. R., Luo, Q., Butler, G., et al., “The calcium binding proteins parvalbumin and calbindin-D 28K form complementary patterns in the cat superior colliculus,” J. Comp. Neurol., 320, 243–256 (1992).
Morand, S., Thut, G., de Peralta, R. G., et al., “Electrophysiological evidence for fast visual processing through the human koniocellular pathway when stimuli move,” Cereb. Cortex, 10, 817–825 (2000).
Mundinano, I.-C., Kwan, W. C., and Bourne, J. A., “Retinotopic specializations of cortical and thalamic inputs to area MT,” Proc. Natl. Acad. Sci. USA, 116, 23,326–23,331 (2019).
Murakami, D. M., Miller, J. D., and Fuller, C. A., “The retinohypothalamic tract in the cat: retinal ganglion cell morphology and pattern of projection,” Brain Res., 482, 283–296 (1989).
Murphy, K. M., Jones, D. G., and Van Sluyters, R. C., “Cytochromeoxidase blobs in cat primary visual cortex,” J. Neurosci., 15, 4196– 4208 (1995).
Nakagawa, S. and Tanaka, S., “Retinal projections to the pulvinar nucleus of the macaque monkey: a re-investigation using autoradiography,” Exp. Brain Res., 57, 151–157 (1984).
Nakamura, H. and Itoh, K., “Cytoarchitectonic and connectional organization of the ventral lateral geniculate nucleus in the cat,” J. Comp. Neurol., 473, 439–462 (2004).
Nassi, J. J. and Callaway, E. M., “Multiple circuits relaying primate parallel visual pathways to the middle temporal area,” J. Neurosci., 26, 12789–12798 (2006).
Nassi, J. J. and Callaway, E. M., “Parallel processing strategies of the primate visual system,” Nat. Rev. Neurosci., 10, 360–372 (2009).
Neitz, J. and Neitz, M., “Evolution of the circuitry for conscious color vision in primates,” Eye (Lond.), 31, 286–300 (2017).
Niimi, K., Miki, M., and Kawamura, S., “Ascending projections of the superior colliculus in the cat,” Okajimas Folia Anat. Jpn., 47, 269–287 (1970).
O’Brien, B. J., Abel, P. L., and Olavarria, J. F., “The retinal input to calbindin-D28k-defi ned subdivisions in macaque inferior pulvinar,” Neurosci. Lett., 312, 145–148 (2001).
Payne, B. R., Lomber, S. G., Macneil, M. A., and Cornwell, P., “Evidence for greater sight in blindsight following damage of primary visual cortex early in life,” Neuropsychologia, 34, 741–774 (1996).
Pearlman, A. L. and Daw, N. W., “Opponent color cells in the cat lateral geniculate nucleus,” Science, 167, 84–86 (1970).
Percival, K. A., Koizumi, A., Masri, R. A., et al., “Identification of a pathway from the retina to koniocellular layer K1 in the lateral geniculate nucleus of marmoset,” J. Neurosci., 34, 3821–3825 (2014).
Perry, V. H. and Cowey, A., “Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey,” Neurosci., 12, 1125–1137 (1984).
Perry, V. H., Oehler, R., and Cowey, A., “Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey,” Neurosci., 12, 1101–1123 (1984).
Pietersen, A. N. J., Cheong, S. K., Solomon, S. G., et al., “Temporal response properties of koniocellular (blue-on and blue-off) cells in marmoset lateral geniculate nucleus,” J. Neurophysiol., 112, 1421–1438 (2014).
Podvigin, N. F., Makarov, F. N., and Shelepin, Yu. E., Elements of the Structural-Functional Organization of the Visual and Oculomotor Systems,” Nauka, Leningrad (1986).
Polyak, S., “Minute structure of the retina in monkeys and in apes,” Arch. Ophthalmol, 15, 477–519 (1936).
Preuss, T. M. and Kaas, J. H., “Cytochrome oxidase ‘blobs’ and other characteristics of primary visual cortex in a lemuroid primate, Cheirogaleus medius,” Brain Behav. Evol., 47, 103–112 (1996).
Pu, M., “Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus,” J. Comp. Neurol., 414, 267–274 (1999).
Raczkowski, D. and Rosenquist, A. C., “Connections of the parvocellular C laminae of the dorsal lateral geniculate nucleus with the visual cortex in the cat,” Brain Res., 199, 447–451 (1980).
Raczkowski, D., Hamos, J. E., and Sherman, S. M., “Synaptic circuitry of physiologically identified W-cells in the cat’s dorsal lateral geniculate nucleus,” J. Neurosci., 8, 31–48 (1988).
Rapaport, D. H., Fletcher, J. T., LaVail, M. M., and Rakic, P., “Genesis of neurons in the retinal ganglion cell layer of the monkey,” J. Comp. Neurol., 322, 577–588 (1992).
Reese, B. E., Thompson, W. F., and Peduzzi, J. D., “Birthdates of neurons in the retinal ganglion cell layer of the ferret,” J. Comp. Neurol., 341, 464–475 (1994).
Rheaume, B. A., Jereen, A., Bolisetty, M., et al., “Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes,” Nat. Commun., 9, 2759 (2018).
Rima, S. and Schmid, M. C., “V1-bypassing thalamo-cortical visual circuits in blindsight and developmental dyslexia,” Curr. Opin. Physiol., 16, 14–20 (2020).
Rockland, K. S., “Anatomical organization of primary visual cortex (area 17) in the ferret,” J. Comp. Neurol., 241, 225–236 (1985).
Rockoff, E., Balaram, P., and Kaas, J., “Patchy distributions of myelin and vesicular glutamate transporter 2 align with cytochrome oxidase blobs and interblobs in the superfi cial layers of the primary visual cortex,” Eye Brain, 6, Supplement 1, 19–27 (2014).
Rodman, H. R., Gross, C. G., and Albright, T. D., “Afferent basis of visual response properties in area MT of the macaque: II. Effects of superior colliculus removal,” J. Neurosci., 10, 1154–1164 (1990).
Rodman, H. R., Sorenson, K. M., Shim, A. J., and Hexter, D. P., “Calbindin immunoreactivity in the geniculo-extrastriate system of the macaque: implications for heterogeneity in the koniocellular pathway and recovery from cortical damage,” J. Comp. Neurol., 431, 168–181 (2001).
Roe, A. W., Garraghty, P. E., Esguerra, M., and Sur, M., “Experimentally induced visual projections to the auditory thalamus in ferrets: evidence for a W cell pathway,” J. Comp. Neurol., 334, 263–280 (1993).
Rowe, M. H. and Cox, J. F., “Spatial receptive-field structure of cat retinal W cells,” Vis. Neurosci., 10, 765–779 (1993).
Rowe, M. H. and Dreher, B., “Retinal W-cell projections to the medial interlaminar nucleus in the cat: implications for ganglion cell classification,” J. Comp. Neurol., 204, 117–133 (1982).
Rowe, M. H. and Palmer, L. A., “Spatio-temporal receptive-field structure of phasic W cells in the cat retina,” Vis. Neurosci., 12, 117–139 (1995).
Saalmann, Y. B. and Kastner, S., “Gain control in the visual thalamus during perception and cognition,” Curr. Opin. Neurobiol., 19, 408– 14 (2009).
Saalmann, Y. B., Pinsk, M. A., Wang Liang, et al., “The pulvinar regulates information transmission between cortical areas based on attention demands,” Science, 337, 753–756 (2012).
Schiller, P. H. and Malpeli, J. G., “Properties and tectal projections of monkey retinal ganglion cells,” J. Neurophysiol., 40, 428–445 (1977).
Schmid, M. C., Mrowka, S. W., Turchi, J., et al., “Blindsight depends on the lateral geniculate nucleus,” Nature, 466, 373–377 (2010).
Schmid, M. C., Panagiotaropoulos, T., Augath, M. A., et al., “Visually driven activation in macaque areas V2 and V3 without input from the primary visual cortex,” PLoS One, 4, e5527 (2009).
Schmidt, T. M., Chen, S.-K., and Hattar, S., “Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions,” Trends Neurosci., 34, 572–580 (2011).
Semo, M., Llamosas, M. M., Foster, R. G., and Jeffery, G., “Melanopsin (Opn4) positive cells in the cat retina are randomly distributed across the ganglion cell layer,” Vis. Neurosci., 22, 111–116 (2005).
Sharma, J., Angelucci, A., and Sur, M., “Induction of visual orientation modules in auditory cortex,” Nature, 404, 841–847 (2000).
Shostak, Y., Ding, Y., Mavity-Hudson, J., and Casagrande, V. A., “Cortical synaptic arrangements of the third visual pathway in three primate species: Macaca mulatta, Saimiri sciureus, and Aotus trivirgatus,” J. Neurosci., 22, 2885–2893 (2002).
Silveira, L. C., Lee, B. B., Yamada, E. S., et al., “Ganglion cells of a short-wavelength-sensitive cone pathway in New World monkeys: morphology and physiology,” Vis. Neurosci., 16, 333–43 (1999).
Sincich, L. C., Park, K. F., Wohlgemuth, M. J., and Horton, J. C., “Bypassing V1: a direct geniculate input to area MT,” Nat. Neurosci., 7, 1123–1128 (2004).
Soares, J. G., Botelho, E. P., and Gattass, R., “Distribution of calbindin, parvalbumin and calretinin in the lateral geniculate nucleus and superior colliculus in Cebus apella monkeys,” J. Chem. Neuroanat., 22, 139–146 (2001).
Solomon, S. G., Lee, B. B., White, A. J. R., et al., “Chromatic organization of ganglion cell receptive fields in the peripheral retina,” J. Neurosci., 25, 4527–4539 (2005).
Spear, P. D., McCall, M. A., and Tumosa, N., “W-and Y-cells in the C layers of the cat’s lateral geniculate nucleus: normal properties and effects of monocular deprivation,” J. Neurosci., 61, 58–73 (1989).
Spear, P. D., Smith, D. C., and Williams, L. L., “Visual receptive-field properties of single neurons in cat’s ventral lateral geniculate nucleus,” J. Neurophysiol., 40, 390–409 (1977).
Sprague, J. M., Levy, J., and DiBerardino, A., and Berlucchi, G., “Visual cortical areas mediating form discrimination in the cat, J., “Comp,” Neurology, 172, 441–488 (1977).
Stanford, L. R. and Sherman, S. M., “Structure/function relationships of retinal ganglion cells in the cat,” Brain Res., 297, 381–386 (1984).
Stanford, L. R., “W-cells in the cat retina: correlated morphological and physiological evidence for two distinct classes,” J. Neurophysiol., 57, 218–244 (1987).
Stanford, L. R., Friedlander, M. J., and Sherman, S. M., “Morphological and physiological properties of geniculate W-cells of the cat: a comparison with X and Y-cells,” J. Neurophysiol., 50, 582–608 (1983).
Stepniewska, I., Qi, H. X., and Kaas, J. H., “Do superior colliculus projection zones in the inferior pulvinar project to MT in primates?” Eur. J. Neurosci., 11, 469–480 (1999).
Stepniewska, I., Qi, H. X., and Kaas, J. H., “Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys,” Vis. Neurosci., 17, 529–549 (2000).
Stone, J. and Hoffmann, K. P., “Very slow-conducting ganglion cells in the cat’s retina: a major, new functional type?” Brain Res., 43, 610–616 (1972).
Stone, J. and Keens, J., “Distribution of small and medium-sized ganglion cells in the cat’s retina,” J. Comp. Neurol., 192, 235–246 (1980).
Stone, J., Dreher, B., and Leventhal, A., “Hierarchical and parallel mechanisms in the organization of visual cortex,” Brain Res., 180, 345–394 (1979).
Sur, M. and Sherman, S. M., “Linear and nonlinear W-cells in C-laminae of the cat’s lateral geniculate nucleus,” J. Neurophysiol., 47, 869–884 (1982).
Sur, M., Garraghty, P. E., and Roe, A. W., “Experimentally induced visual projections into auditory thalamus and cortex,” Science, 242, 1437–1441 (1988).
Szmajda, B. A., Grünert, U., and Martin, P. R., “Retinal ganglion cell inputs to the koniocellular pathway,” J. Comp. Neurol., 510, 251–268 (2008).
Tailby, C., Szmajda, B. A., Buzás, P., et al., “Transmission of blue (S) cone signals through the primate lateral geniculate nucleus,” J. Physiol., 586, 5947-5967 (2008).
Telkes, I., Distler, C., and Hoffmann, K. P., “Retinal ganglion cells projecting to the nucleus of the optic tract and the dorsal terminal nucleus of the accessory optic system in macaque monkeys,” Eur. J. Neurosci., 12, 2367-2375 (2000).
Tootell, R. B., Hamilton, S. L., and Silverman, M. S., “Topography of cytochrome oxidase activity in owl monkey cortex,” J. Neurosci., 5, 2786–2800 (1985).
Tootell, R. B., Silverman, M. S., Hamilton, S. L., et al., “Functional anatomy of macaque striate cortex. III. Color,” J. Neurosci., 8, 1569–1593 (1988).
Torrealba, F., Partlow, G. D., and Guillery, R. W., “Organization of the projection from the superior colliculus to the dorsal lateral geniculate nucleus of the cat,” Neurosci., 6, 1341–1360 (1981).
Troy, J. B. and Shou, T., “The receptive fields of cat retinal ganglion cells in physiological and pathological states: where we are after half a century of research,” Prog. Retin. Eye Res., 21, 263–302 (2002).
Turner, E. C., Gabi, M., Liao, C.-C., and Kaas, J. H., “The postnatal development of MT, V1, LGN, pulvinar and SC in prosimian galagos (Otolemur garnettii),” J. Comp. Neurol., 528, 3075–3094 (2020).
Updyke, B. V., “The patterns of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat,” J. Comp. Neurol., 163, 377–395 (1975).
Usrey, W. M. and Reid, R. C., “Visual physiology of the lateral geniculate nucleus in two species of new world monkey: Saimiri sciureus and Aotus trivirgatis,” J. Physiol., 523, 755–769 (2000).
Valverde Salzmann, M. F., Bartels, A., Logothetis, N. K., and Schüz, A., “Color blobs in cortical areas V1 and V2 of the new world monkey Callithrix jacchus, revealed by non-differential optical imaging,” J. Neurosci., 32, 7881–7894 (2012).
Van Essen, D. C., Anderson, C. H., and Felleman, D. J., “Information processing in the primate visual system: an integrated systems perspective,” Science, 255, 419–423 (1992).
Vaney, D. I., “Territorial organization of direction-selective ganglion cells in rabbit retina,” J. Neurosci., 14, 6301–6316 (1994).
Walsh, C., Polley, E. H., Hickey, T. L., and Guillery, R. W., “Generation of cat retinal ganglion cells in relation to central pathways,” Nature, 302, 611–614 (1983).
Warner, C. E., Goldshmit, Y., and Bourne, J. A., “Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei,” Front. Neuroanat., 12, 8 (2010).
Wässle, H., “ Morphological types and central projections of ganglion cells in the cat retina,” Prog. Retin. Res., 1, 125–152 (1982).
Wässle, H., “Parallel processing in the mammalian retina,” Nat. Rev. Neurosci., 5, 747–757 (2004).
Watanabe, M. and Rodieck, R. W., “Parasol and midget ganglion cells of the primate retina,” J. Comp. Neurol., 289, 434–454 (1989).
Weber, J. T., Huerta, M. F., Kaas, J. H., and Harting, J. K., “The projections of the lateral geniculate nucleus of the squirrel monkey: studies of the interlaminar zones and the S layers,” J. Comp. Neurol., 213, 135–145 (1983).
Weiskrantz, L., Warrington, E. K., Sanders, M. D., and Marshall, J., “Visual capacity in the hemianopic field following a restricted occipital ablation,” Brain, 97, 709–728 (1974).
Weller, R. E. and Kaas, J. H., “Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates,” Vis. Neurosci., 3, 327–349 (1989).
White, A. J., Solomon, S. G., and Martin, P. R., “Spatial properties of koniocellular cells in the lateral geniculate nucleus of the marmoset Callithrix jacchus,” J. Physiol., 533, 519–535 (2001).
Wikler, K. C. and Rakic, P., “Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates,” J. Neurosci., 10, 3390–3401 (1990).
Williams, R. W., Cavada, C., and Reinoso-Suárez, F., “Rapid evolution of the visual system: a cellular assay of the retina and dorsal lateral geniculate nucleus of the Spanish wildcat and the domestic cat,” J. Neurosci., 13, 208–228 (1993).
Wilson, P. D. and Stone, J., “Evidence of W-cell input to the cat’s visual cortex via the C laminae of the lateral geniculate nucleus,” Brain Res., 92, 472–478 (1975).
Wilson, P. D., Rowe, M. H., and Stone, J., “Properties of relay cells in cat’s lateral geniculate nucleus: a comparison of W-cells with X and Y-cells,” J. Neurophysiol., 39, 1193–1209 (1976).
Wong-Riley, M. T. T., Hevner, R. F., Cutlan, R., et al., “Cytochrome oxidase in the human visual cortex: distribution in the developing and the adult brain,” Vis. Neurosci., 10, 41–58 (1993).
Xu, X., Bonds, A. B., and Casagrande, V. A., “Modeling receptive-field structure of koniocellular, magnocellular, and parvocellular LGN cells in the owl monkey (Aotus trivigatus),” Vis. Neurosci., 19, 703–711 (2002).
Xu, X., Ichida, J. M., Allison, J. D., Boyd, J. D., et al., “A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotis trivirgatis),” J. Physiol., 531, 203–218 (2001).
Xu, Y., Dhingra, N. K., Smith, R. G., and Sterling, P., “Sluggish and brisk ganglion cells detect contrast with similar sensitivity,” J. Neurophysiol., 93, 2388–2395 (2005).
Xue, J. T., Kim, C. B., Moore, R. J., and Spear, P. D., “Influence of the superior colliculus on responses of lateral geniculate neurons in the cat,” Vis. Neurosci., 11, 1059–1076 (1994).
Yabuta, N. H. and Callaway, E. M., “Functional streams and local connections of layer 4C neurons in primary visual cortex of the macaque monkey,” J. Neurosci., 18, 9489–9499 (1998).
Yan, Y. H., Winarto, A., Mansjoer, I., and Hendrickson, A., “Parvalbumin, calbindin, and calretinin mark distinct pathways during development of monkey dorsal lateral geniculate nucleus,” J. Neurobiol., 31, 189– 209 (1996).
Yang, G. and Masland, R. H., “Direct visualization of the dendritic and receptive fields of directionally selective retinal ganglion cells,” Science, 258, 1949–1952 (1992).
Yukie, M. and Iwai, E., “Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys,” J. Comp. Neurol., 201, 81–97 (1981).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 71, No. 6, pp. 785–802, November–December, 2021.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Merkulyeva, N.S. Conducting Channels in the Visual System. The Third Channel. Neurosci Behav Physi 52, 886–898 (2022). https://doi.org/10.1007/s11055-022-01313-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-022-01313-4