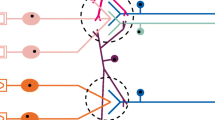
The phenomenal sensitivity of the olfactory system and its ability to detect an enormous diversity of olfactory stimuli are to a large extent due to the existence of numerous olfactory receptors. The genes encoding olfactory receptors (OR) form the largest family in mammalian genomes. The defining principle in the model of olfactory information encoding is the “one neuron–one receptor” rule, whereby just one gene of the OR gene family is selected for expression in each of the 107 olfactory neurons located in the olfactory epithelium. Although the physiological appropriateness of the “one neuron–one receptor” principle is undoubted, the rule is essentially hypothetical in nature and has not been verified experimentally to the necessary level. In particular, the question of the epigenetic mechanisms of stochastic, monogenic, and monoallelic expression of OR genes remains open. The results of deep sequencing of the transcriptomes of single cells unexpectedly revealed multireceptor neurons containing transcripts for several OR genes. Does this mean that the rule which more than 25 years ago was believed to be physiologically confirmed, unshakeable, and unarguable does not operate? This review will present different interpretations of results from studies of the transcriptomes of single olfactory neurons which explain the existence of “multireceptor” neurons and their role in the process of olfactory neurogenesis.
Similar content being viewed by others
References
A. W. Barrios, P. Sánchez-Quinteiro, and I. Salazar, “Dog and mouse: toward a balanced view of the mammalian olfactory system,” Front. Neuroanat., 8, 106 (2014).
K. Bleymehl, A. Pérez-Gómez, M. Omura, et al., “A sensor for low environmental oxygen in the mouse main olfactory epithelium,” Neuron, 92, 1196–1203 (2016).
H. Breer, J. Fleischer, and J. Strotmann, “The sense of smell: multiple olfactory subsystems,” Cell. Mol. Life Sci., 63, 1465–1475 (2006).
P. A. Brennan and F. Zufall, “Pheromonal communication in vertebrates,” Nature, 444, 308–315 (2006).
L. Buck and R. Axel, “A novel multigene family may encode odorant receptors: a molecular basis for odor recognition,” Cell, 65, 175–187 (1991).
R. P. Dalton, D. B. Lyons, and S. Lomvardas, “Co-opting the unfolded protein response to elicit olfactory receptor feedback,” Cell, 155, 321–332 (2013).
C. Flegel, S. Manteniotis, S. Osthold, et al., “Expression profile of ectopic olfactory receptors determined by deep sequencing,” PLoS One, 8, e55368 (2013).
G. Glusman, I. Yanai, I. Rubin, and D. Lancet, “The complete human olfactory subgenome,” Genome Res., 11, 685–702 (2001).
J. A. Gogos, J. Osborne, A. Nemes, et al., “Genetic ablation and restoration of the olfactory topographic map,” Cell, 103, 609–620 (2000).
P. P. Graziadei and G. A. Monti Graziadei, “Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons,” J. Neurocytol., 8, 1–18 (1979).
N. K. Hanchate, K. Kondoh, Z. Lu, et al., “Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis,” Science, 350, 1251–1255 (2015).
T. Imai, H. Sakano, and L. B. Vosshall, “Topographic mapping – the olfactory system,” Cold Spring Harb. Perspect. Biol., 2, a001776 (2010).
T. Ishii, S. Serizawa, A. Kohda, et al., “Monoallelic expression of the odorant receptor gene and axonal projection of olfactory sensory neurons,” Genes Cells, 6, 71–78 (2001).
Y. Jiang and H. Matsunami, “Mammalian odorant receptors: functional evolution and variation,” Curr. Opin. Neurobiol., 34, 54–60 (2015).
M. A. Johnson, L. Tsai, D. S. Roy, et al., “Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem,” Proc. Natl. Acad. Sci. USA, 109, 13410–13415 (2012).
M. Kuhn, “Molecular physiology of membrane guanylyl cyclase receptors,” Physiol. Rev., 96, 751–804 (2016).
T. Leinders-Zufall, R. E. Cockerham, S. Michalakis, et al., “Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium,” Proc. Natl. Acad. Sci. USA, 104, 14507–14512 (2007).
J. W. Lewcock and R. R. Reed, “A feedback mechanism regulates monoallelic odorant receptor expression,” Proc. Natl. Acad. Sci. USA, 101, 1069–1074 (2004).
Q. Li, “Deorphanization of olfactory trace amine-associated receptors,” Meth. Mol. Biol., 1820, 21–31 (2018).
T. Liberia, E. Martin-Lopez, S. J. Meller, and C. A. Greer, “Sequential maturation of olfactory sensory neurons in the mature olfactory epithelium,” eNeuro, 6, P.ENEURO.0266-19 (2019).
S. D. Liberles, “Trace amine-associated receptors: ligands, neural circuits, and behaviors,” Curr. Opin. Neurobiol., 34, 1–7 (2015).
S. Lomvardas, G. Barnea, D. J. Pisapia, et al., “Interchromosomal interactions and olfactory receptor choice,” Cell, 126, 403–413 (2006).
A. Mackay-Sim and P. W. Kittel, “On the life span of olfactory receptor neurons,” Eur. J. Neurosci., 3, 209–215 (1991).
B. Malnic, J. Hirono, T. Sato, and L. B. Buck, “Combinatorial receptor codes for odors,” Cell, 96, 713–723 (1999).
E. Markenscoff-Papadimitriou, W. E. Allen, B. M. Colquitt, et al., “Enhancer interaction networks as a means for singular olfactory receptor expression,” Cell, 159, 543–557 (2014).
F. Moine, J. Brechbühl, M. Nenniger Tosato, et al., “Alarm pheromone and kairomone detection via bitter taste receptors in the mouse Grueneberg ganglion,” BMC Biol., 16, 12 (2018).
P. Mombaerts, “Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited,” Curr. Opin. Neurobiol., 14, 31–36 (2004).
P. Mombaerts, “Axonal wiring in the mouse olfactory system,” Ann. Rev. Cell. Dev. Biol., 22, 713–737 (2006).
K. Monahan, A. Horta, and S. Lomvardas, “LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice,” Nature, 565, 448–453 (2019).
K. Monahan, I. Schieren, and J. Cheung, et al., “Cooperative interactions enable singular olfactory receptor expression in mouse olfactory neurons,” eLife, 21, 6 (2017).
E. E. Morrison and R. M. Costanzo, “Morphology of olfactory epithelium in humans and other vertebrates,” Microsc. Res. Tech., 23, 49–61 (1992).
D. G. Moulton, “Spatial patterning of response to odors in the peripheral olfactory system,” Physiol. Rev., 56, 578–593 (1976).
S. D. Munger, T. Leinders-Zufall, L. M. McDougall, et al., “An olfactory subsystem that detects carbon disulfi de and mediates food-related social learning,” Curr. Biol., 20, 1438–1444 (2010).
T. Nakamura and G. H. Gold, “A cyclic nucleotide-gated conductance in olfactory receptor cilia,” Nature, 325, 442–444 (1987).
K. Narusuye, F. Kawai, and E.-I. Miyachi, “Spike encoding of olfactory receptor cells,” Neurosci. Res., 46, 407–413 (2003).
Y. Niimura, A. Matsui, and K. Touhara, “Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals,” Genome Res., 24, 1485–1496 (2014).
Y. Niimura and M. Nei, “Extensive gains and losses of olfactory receptor genes in mammalian evolution,” PLoS One, 2, e708 (2007).
M. Omura and P. Mombaerts, “Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse,” Cell Rep., 8, 583–595 (2014).
M. Omura and P. Mombaerts, “Trpc2-expressing sensory neurons in the mouse main olfactory epithelium of type B express the soluble guanylate cyclase Gucy1b2,” Mol. Cell. Neurosci., 6, 114–124 (2015).
S. Pifferi, V. Cenedese, and A. Menini, “Anoctamin2/TMEM16B: a calcium-activated chloride channel in olfactory transduction,” Exp. Physiol., 97, 193–199 (2001).
K. J. Ressler, S. L. Sullivan, and L. B. Buck, “A zonal organization of odorant receptor gene expression in the olfactory epithelium,” Cell, 73, 597–609 (1993).
K. J. Ressler, S. L. Sullivan, and L. B. Buck, “Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb,” Cell, 79, 1245–1255 (1994).
L. R. Saraiva, X. Ibarra-Soria, and K. Khan, et al., “Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq,” Sci. Rep., 5, 18178 (2015).
J. E. Schwob, W. Jang, and E. H. Holbrook, et al., “Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license,” J. Comp. Neurol., 525, 1034–1054 (2017).
E. Schultz, “Repair of the olfactory mucosa with special reference to regeneration of olfactory cells (sensory neurons),” Am. J. Pathol., 37, 1–19 (1960).
S. Serizawa, K. Miyamichi, H. Nakatani, et al., “Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse,” Science, 302, 2088–2094 (2003).
S. Serizawa, K. Miyamichi, and H. Sakano, “One neuron-one receptor rule in the mouse olfactory system,” Trends Genet., 20, 648–653 (2004).
B. M. Shykind, S. C. Rohani, S. O’Donnell, et al., “Gene switching and the stability of odorant receptor gene choice,” Cell, 117, 801–815 (2004).
C. G. Smith, “Regeneration of sensory olfactory epithelium and nerves in adult frogs,” Anat. Rec., 109, 661–671 (1951).
L. Tan, Q. Li, and X. S. Xie, “Olfactory sensory neurons transiently express multiple olfactory receptors during development,” Mol. Syst. Biol., 11, 844 (2015).
L. Tan, D. Xing, N. Daley, and X. S. Xie, “Three-dimensional genome structures of single sensory neurons in mouse visual and olfactory systems,” Nat. Struct. Mol. Biol., 26, 297–307 (2019).
R. Vassar, S. K. Chao, and R. Sitcheran, “Topographic organization of sensory projections to the olfactory bulb,” Cell, 79, 981–991 (1994).
R. Vassar, J. Ngai, and R. Axel, “Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium,” Cell, 74, 309–318 (1993).
S. L. Youngentob, P. F. Kent, and F. L. Margolis, “OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns,” J. Neurophysiol, 90, 3864–3873 (2003).
X. Zhang, X. Zhang, and S. Firestein, “Comparative genomics of odorant- and pheromone receptor genes in rodents,” Genomics, 89, 441–450 (2007).
J. Zheng and W. N. Zagotta, “Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels,” Neuron, 42, 411–421 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Uspekhi Fiziologicheskikh Nauk, Vol. 51, No. 3, pp. 3–15, July–September, 2021.
Rights and permissions
About this article
Cite this article
Bystrova, M.F., Kolesnikov, S.S. The “One Neuron–One Receptor” Rule in the Physiology and Genetics of Olfaction. Neurosci Behav Physi 51, 1008–1017 (2021). https://doi.org/10.1007/s11055-021-01159-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-021-01159-2