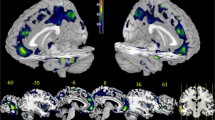
Objectives. To identify the neuroanatomical characteristics of the gray matter in individual areas of the prefrontal cortex (PFC) and a number of subcortical formations in patients with juvenile shiftlike schizophrenia (ICD-10, F20) Materials and methods. A total of 43 patients and 54 mentally healthy men, mean age 22 years, were studied. The main methods were psychopathological investigations and MRI brain scans producing high-resolution T1-weighted images. Results. As compared with the control group, the group of patients with schizophrenia showed a decrease in the thickness of the gray matter in all segments of the prefrontal cortex studied, though no between-group differences in the volume of the subcortical formations were seen. No statistically significant correlations between structural changes and measures of the severity of psychopathological disorders were found. Conclusions. The data obtained from these studies show that structural anomalies in the frontal areas of the brain in juvenile shiftlike schizophrenia are not linked with the severity of psychopathological symptoms.
Similar content being viewed by others
References
T. G. Van Erp, D. P. Hibar, J. M. Rasmussen, et al., “Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium,” Mol. Psychiatry, 21, No. 4, 547–553 (2016), https://doi.org/10.1038/mp.2015.63.
T. G. M. van Erp, E. Walton, D. P. Hibar, et al., “Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium,” Biol. Psychiatry, 18, 31517–31518 (2018).
R. McCarley, C. Wible, M. Frumin, et al., “MRI anatomy of schizophrenia,” Biol. Psychiatry, 45, No. 9, 1099–1119 (1999), https://doi.org/10.1016/s0006-3223(99)00018-9.
D. R. Weinberger, “Schizophrenia, the prefrontal cortex, and a mechanism of genetic susceptibility,” Eur. Psychiatry, 17(Suppl. 4), 355–362 (2002), https://doi.org/10.1016/s0924-9338(03)00080-4.
T. Sakurai, N. Gamo, T. Hikida, et al., “Converging models of schizophrenia – network alterations of prefrontal cortex underlying cognitive impairments,” Prog. Neurobiol., 134, 178–201 (2015), https://doi.org/10.1016/j.pneurobio.2015.09.010.
V. M. Goghari, A. W. Macdonald 3rd, and S. R. Sponheim, “Relationship between prefrontal gray matter volumes and working memory performance in schizophrenia: a family study,” Schizophr. Res., 153, No. 1–3, 113–121 (2014), https://doi.org/10.1016/j.schres.2014.01.032.
J. J. Levitt, L. Bobrow, D. Lucia, and P. Srinivasan, “A selective review of volumetric and morphometric imaging in schizophrenia,” Curr. Top. Behav. Neurosci., 4, 243–281 (2010), https://doi.org/10.1007/7854_2010_53.
B. Brent, H. Thermenos, M. Keshavan, and L. Seidman, “Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: A review of structural MRI findings,” Child Adolesc. Psychiatr. Clin. N. Am., 22, No. 4, 689–714 (2013), https://doi.org/10.1016/j.chc.2013.06.003.
B. Dietsche, T. Kircher, and I. Falkenberg, “Structural brain changes in schizophrenia at different stages of the illness: A selective review of longitudinal magnetic resonance imaging studies,” Aust. N. Z. J. Psychiatry, 51, No. 5, 500–508 (2017), https://doi.org/10.1177/0004867417699473.
H. Witthaus, C. Kaufmann, G. Bohner, et al., “Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls,” Psychiatry Res., 173, No. 3, 163–169 (2009), https://doi.org/10.1016/j.pscychresns.2008.08.002.
H. W. Thermenos, M. S. Keshavan, R. J. Juelich, et al., “A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia,” Am J. Med. Genet. B Neuropsychiatr. Genet., 162B, No. 7, 604–635 (2013), https://doi.org/10.1002/ajmg.b.32170.
W. Zhang, W. Deng, L. Yao, et al., “Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia,” Am. J. Psychiatry, 172, No. 10, 995–1003 (2015), https://doi.org/10.1176/appi.ajp.2015.14091108.
T. Ohtani, E. Del Re, J. J. Levitt, et al., “Progressive symptom-associated prefrontal volume loss occurs in first-episode schizophrenia but not in affective psychosis,” Brain Struct. Funct., 223, No. 6, 2879–2892 (2018), https://doi.org/10.1007/s00429-018-1634-0.
M. Chang, F. Y. Womer, C. Bai, et al., “Voxel-based morphometry in individuals at genetic high risk for schizophrenia and patientswith schizophrenia during their first episode of psychosis,” PLoS One, 11, No. 10, e0163749 (2016), https://doi.org/10.1371/journal.pone.0163749.
M. E. Shenton, C. C. Dickey, M. Frumin, and R. W. McCarley, “A review of MRI findings in schizophrenia,” Schizophr. Res., 49, No. 1–2, 1–52 (2001), https://doi.org/10.1016/s0920-9964(01)00163-3.
A. M. Shepherd, K. R. Laurens, S. L. Matheson, et al., “Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia,” Neurosci. Biobehav. Rev., 36, No. 4, 1342–1356 (2012), https://doi.org/10.1016/j.neubiorev.2011.12.015.
V. G. Kaleda, I. S. Lebedeva, A. O. Yakimov, et al., “Structuralfunctional characteristics of the brain in juvenile patients in remission after a first episode of endogenous psychosis,” Zh. Nevrol. Psikhiat., 111, No. 10, 18–22 (2011).
B. Fischl, “Free Surfer,” Neuroimage, 62, 774–781 (2012), https://doi.org/10.1016/j.neuroimage.2012.01.021.
B. Fischl, D. H. Salat, E. Busa, et al., “Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain,” Neuron, 33, 341–355 (2002), https://doi.org/10.1016/s0896-6273(02)00569-x.
B. Fischl, D. H. Salat, A. J. van der Kouwe, et al., “Sequence-independent segmentation of magnetic resonance images,” Neuroimage, 23, 69–84 (2004), https://doi.org/10.1016/s0896-6273(02)00569-x.
F. Segonne, A. M. Dale, E. Busa, et al., “A hybrid approach to the skull stripping problem in MRI,” Neuroimage, 22, 1060–1075 (2004), https://doi.org/10.1016/j.neuroimage.2004.03.032.
A. M. Dale and M. I. Sereno, “Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach,” J. Cogn. Neurosci., 5, 162–176 (1993), https://doi.org/10.1162/jocn.1993.5.2.162.
A. M. Dale, B. Fischl, and M. I. Sereno, “Cortical surface-based analysis. I. Segmentation and surface reconstruction,” Neuroimage,9, 179–794 (1999), https://doi.org/10.1006/nimg.1998.0395.
B. Fischl, M. I. Sereno, and A. M. Dale, “Cortical surface-based analysis. II: Infl ation, fl attening, and a surface-based coordinate system,” Neuroimage, 9, 195–207 (1999).
B. Fischl, A. van der Kouwe, C. Destrieux, et al., “Automatically parcellating the human cerebral cortex,” Cereb. Cortex, 14, 11–22 (2004), https://doi.org/10.1093/cercor/bhg087.
R. S. Desikan, F. Segonne, B. Fischl, et al., “An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest,” Neuroimage, 31, 968–980 (2006), https://doi.org/10.1016/j.neuroimage.2006.01.021.
S. Holm, “A simple sequentially rejective multiple test procedure,” Scand. J. Statistics, 6, 65–70 (1979).
R. Wolf, A. Hose, K. Frasch, et al., “Volumetric abnormalities associated with cognitive defi cits in patients with schizophrenia,” Eur. Psychiatry, 23, 541–548 (2008), https://doi.org/10.1016/j.eurpsy.2008.02.002.
H. Witthaus, C. Kaufmann, G. Bohner, et al., “Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls,” Psychiatry Res., 173, No. 3, 163–169 (2009), https://doi.org/10.1016/j.pscychresns.2008.08.002.
N. C. Andreasen, D. Liu, S. Ziebell, et al., “Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: A prospective longitudinal MRI study,” Am. J. Psychiatry, 170, No. 6, 609–615 (2013), https://doi.org/10.1176/appi.ajp.2013.12050674.
Y. Yue, L. Kong, J. Wang, et al., “Regional abnormality of grey matter in schizophrenia: Effect from the illness or treatment?” PLoS One, 11, No. 1, e0147204 (2016), https://doi.org/10.1371/journal.pone.0147204.
L. Tomelleri, J. Jogia, C. Perlini, et al., Neuroimaging Network of the ECNP networks initiative, “Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia,” Eur. Neuropsychopharmacol., 19, No. 12, 835–840 (2009), https://doi.org/10.1016/j.euroneuro.2009.07.007.
J. A. Lieberman, G. D. Tollefson, C. Charles, et al., HGDH Study Group, “Antipsychotic drug effects on brain morphology in first-episode psychosis,” Arch. Gen. Psychiatry, 62, No. 4, 361–370 (2005), https://doi.org/10.1001/archpsyc.62.4.361.
U. S. Torres, F. L. Duran, M. S. Schaufelberger, et al., “Patterns of regional gray matter loss at different stages of schizophrenia: A multisite, crosssectional VBM study in first-episode and chronic illness,” NeuroImage Clin, 12, 1–15 (2016), https://doi.org/10.1016/j.nicl.2016.06.002.
G. Boonstra, W. Cahn, H. G. Schnack, et al., “Duration of untreated illness in schizophrenia is not associated with 5-year brain volume change,” Schizophr. Res., 132, No. 1, 84–90 (2011), https://doi.org/10.1016/j.schres.2011.07.018.
S. Lui, W. Deng, X. Huang, et al., “Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study,” Am. J. Psychiatry, 166, No. 2, 196–205 (2009), https://doi.org/10.1176/appi.ajp.2008.08020183.
N. Koutsouleris, C. Gaser, M. Jager, et al., “Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study,” Neuroimage, 39, No. 4, 1600–1612 (2008), https://doi.org/10.1016/j.neuroimage.2007.10.029.
E. Walton, D. P. Hibar, T. G. M. van Erp, et al., “Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium,” Psychol. Med., 48, No. 1, 82–94 (2018), https://doi.org/10.1017/s0033291717001283.
J. L. Padmanabhan, N. Tandon, C. S. Haller, et al., “Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders,” Schizophr. Bull., 41, No. 1, 154–162 (2015), https://doi.org/10.1093/schbul/sbu075.
I. I. Gottesman and T. D. Gould, “The endophenotype concept in psychiatry: etymology and strategic intentions,” Am. J. Psychiatry, 160, No. 4, 636–645 (2003), https://doi.org/10.1176/appi.ajp.160.4.636.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 119, No. 8, Iss. 1, pp. 7–11, August, 2019.
Rights and permissions
About this article
Cite this article
Kaleda, V.G., Bozjko, O.V., Akhadov, T.A. et al. Neuroanatomical Features of the Brain in Juvenile Shiftlike Schizophrenia: Morphometry of the Gray Matter of the Prefrontal Cortex and Subcortical Structures. Neurosci Behav Physi 50, 541–545 (2020). https://doi.org/10.1007/s11055-020-00934-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00934-x