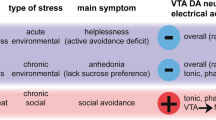
Depression in the biological models considered here can be split into two opposite patterns of changes in serotonin and dopamine levels, corresponding to passive and active stress response tactics [Selye, 1979] – ↑5HT, ↓DA and ↓5HT, ↑DA. Unavoidable pain stimulation, chronic moderate aversive stimulation, unavoidable social suppression, and learned helplessness in response to social defeats are characterized by the ↑5HT, ↓DA pattern. Aggressive behavior to an intruder, avoidance of an aggressive inhabitant, avoidance of pain stimulation, and injection of ketamine all activate the ↓5HT, ↑DA pattern. The physiological nucleus of learned helplessness and social avoidance may be the Pavlovian conditioned freezing reflex in response to a threat and the conditioned avoidance reaction, respectively. Further studies should address the following questions: how does neuron activity in the raphe nuclei change during forced swimming? Why does the increase in the serotonin level in response to antidepressants increase swimming while serotonin neurons are inhibited during swimming? Why is the increase in the serotonin level in the state of learned helplessness combined with suppression of active swimming?
Similar content being viewed by others
References
Abbasi, J., “Ketamine minus the trip: new hope for treatment-resistant depression,” JAMA, 318, No. 20, 1964–1966 (2017).
Admon, R. and Pizzagalli, D. A., “Dysfunctional reward processing in depression,” Curr. Opin. Psychol., 4, 114–118 (2015).
Aghajanian, G. K. and Sanders-Bush, E., Neuropsychopharmacology: The Fifth Generation of Progress, Chpt. 2, Serotonin (2002) pp. 15–34.
Amat, J., Aleksejev, R. M., Paul, E., et al., “Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat,” Neuroscience, 165, 1031–1038 (2010).
Amat, J., Baratta, M. V., Paul, E., et al., “Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus,” Nat. Neurosci., 8, No. 3, 365–371 (2005).
Amat, J., Dolzani, S. D., Tilden, S., et al., “Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress,” J. Neurosci., 36, No. 1, 153–161 (2016).
Amat, J., Paul, E., Watkins, L. R., and Maier, S. F., “Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control,” Neuroscience, 154, 1178–1186 (2008).
Artigas, F. and Bortolozzi, A., “Therapeutic potential of conjugated siRNAs for the treatment of major depressive disorder,” Neuropsychopharmacology, 42, No. 1, 371 (2017).
Barch, D. M., Pagliaccio, D., and Luking, K., “Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia,” Curr. Top. Behav. Neurosci., 27, 411–49 (2016).
Bath, K. G., Russo, S. J., Pleil, K. E., et al., “Circuit and synaptic mechanisms of repeated stress: Perspectives from differing contexts, duration, and development,” Neurobiol. Stress, 7, 137–151 (2017).
Belujon, P. and Grace, A. A., “Dopamine system dysregulation in major depressive disorders,” Int. J. Neuropsychopharmacol., 20, No. 12, 1036–1046 (2017).
Belujon, P. and Grace, A. A., “Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity,” Biol. Psychiatry, 76, 927–936 (2014).
Berridge, K. C. and Robinson, T. E., “Liking, wanting, and incentive sensitization theory of addiction,” Am. Psychol., 71, No. 8, 670–679 (2016).
Berridge, K. C. and Robinson, T. E., “What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience,” Brain Res. Rev., 28, No. 3, 309–69 (1998).
Bortolozzi, A., Castane, A., Semakova, J., et al., “Selective siRNA-mediated suppression of 5HT1A autoreceptors evokes strong antidepressant-like effects,” Mol. Psychiatry, 17, No. 6, 612–623 (2012).
Bosker, F. J., Cremers, T. I. F. H., Jongsma, M. E., et al., “Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine1A receptor- mediated feedback: a microdialysis study in the amygdale,” J. Neurochem., 76, 1645–1653 (2001).
Boureau, Y.-L. and Dayan, P., “Opponency revisited: competition and cooperation between dopamine and serotonin,” Neuropsychopharmacology, 36, No. 1, 74–97 (2011).
Bruzos-Cidon, C., Miguelez, C., Rodríguez, J. J., et al., “Altered neuronal activity and differential sensitivity to acute antidepressants of locus coeruleus and dorsal raphe nucleus in Wistar Kyoto rats: A comparative study with Sprague Dawley and Wistar rats,” Eur. Neuropsychopharmacol., 24, 1112–1122 (2014).
Burghardt, N. S., and Bauer, E. P., “Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits,” Neuroscience, 247, 253–272 (2013).
Burghardt, N. S., Bush, D. E. A., McEwen, B. S., and LeDoux, J. E., “Acute SSRIs increase conditioned fear expression: blockade with a 5HT2C receptor antagonist,” Biol. Psychiatry, 62, No. 10, 1111–1118 (2007).
Campese, V. D., Sears, R. M., Moscarello, J. M., et al., “The neural foundations of reaction and action in aversive motivation,” Curr. Top. Behav. Neurosci., 27, 171–195 (2016).
Carhart-Harris, R. L. and Nutt, D. J., “Serotonin and brain function: a tale of two receptors,” J. Psychopharmacol., 31, No. 9, 1091–1120 (2017).
Challis, C. and Berton, O., “Top-down control of serotonin systems by the prefrontal cortex: a path towards restored socioemotional function in depression,” ACS Chem. Neurosci., 6, No. 7, 1040–1054 (2015).
Challis, C., Beck, S. G., and Berton, O., “Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat,” Front. Behav. Neurosci., 8, Art. 43, 1–14 (2014).
Chaudhury, D., Liu, H., and Han, M.-H., “Neuronal correlates of depression,” Cell. Mol. Life Sci., 72, No. 24, 4825–4848 (2015).
Chaudhury, D., Walsh, J. J., Friedman, A. K., et al., “Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons,” Nature, 493, No. 7433, 532–536 (2013).
Cowen, P. J. and Browning, M., “What has serotonin to do with depression?” World Psychiatry, 14, No. 2, 158–160 (2015).
Darvas, M., Fadok, J. P., and Palmiter, R. D., “Requirement of dopamine signaling in the amygdale and striatum for learning and maintenance of a conditioned avoidance response,” Learn. Mem., 18, 136–143 (2011).
Davis, J. M., Giakas, W. J., Qu, J., et al., “Should we treat depression with drugs or psychological interventions? A reply to Ioannidis,” Philos. Ethics Hum. Med., 6, 8 (2011).
De Kloet, E. R. and Molendijk, M. L., “Coping with the forced swim stressor: towards understanding an adaptive mechanism,” Neural Plast., 2016, Art. ID 6503162.
Dolzani, S. D., Baratta, M. V., Amat, J., et al., “Activation of a habenulo-raphe circuit is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat,” eNeuro, 3, No. 5, 1–17 (2016).
Duman, R. S., Aghajanian, G. K., Sanacora, G., and Krystal, J. H., “Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants,” Nat. Med., 22, No. 3, 238–249 (2016).
Fischer, A. G. and Ullsperger, M., “An update on the role of serotonin and its interplay with dopamine for reward,” Front. Hum. Neurosci., 11, 1–10 (2017).
Fonseca, M. S., Murakami, M., and Mainen, Z. F., “Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing,” Curr. Biol., 25, 1–10 (2015).
Forgeard, M. J. C., Haigh, E. A. P., Beck, A. T., et al., “Beyond depression: towards a process-based approach to research, diagnosis, and treatment,” Clin, Psychol., 18, No. 4, 275–299 (2011).
Fuchicami, F., Thomas, A., Liu, R., et al., “Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions,” Proc. Natl. Acad. Sci. USA, 112, No. 26, 8106–8111 (2015).
Galyamina, A. G., Kovalenko, I. L., Smagin, D. A., and Kudryavtseva, N. N., “Interaction of depression and anxiety in the development of mixed anxious-depressive disorder. Experimental studies of the mechanisms of comorbidity,” Zh. Vyssh. Nerv. Deyat., 66, No. 2, 181–201 (2016).
Golden, S. A., Covington III, H. E., Berton, O., and Russo, S. J., “A standardized protocol for repeated social defeat stress in mice,” Nat. Protoc., 6, No. 8, 1183–1191 (2011).
Goncharov, I. A., Oblomov, GIKhL, Moscow (1953).
Grigor’yan, G. A. and Gulyaeva, N. V., “Modeling of depression in animals: behavior as the basis of a methodology and assessment and classification criteria,” Zh. Vyssh. Nerv. Deyat., 65, No. 6, 643–660 (2015).
Hajós, M., Hoffmann, W. E., and Weaver, R. J., “Regulation of septo-hippocampal activity by 5-hydroxytryptamine2C receptors,” J. Pharmacol. Exp. Ther., 306, No. 2, 605–615 (2003).
Hamani, C., Diwan, M., Macedo, C. E., et al., “AntidepressantLike effects of medial prefrontal cortex deep brain stimulation in rats,” Biol. Psychiatry, 67, 117–124 (2010).
Hervás, I., Queiroz, C. M. T., Adell, A., and Artigas, F., “Role of uptake inhibition and autoreceptor activation in the control of 5HT release in the frontal cortex and dorsal hippocampus of the rat,” Brit. J. Pharmacol., 130, 160–166 (2000).
Heshmati, M. and Russo, S. J., “Anhedonia and the brain reward circuitry in depression,” Curr. Behav. Neurosci. Rep., 2, No. 3, 146–153 (2015).
Hjorth, S., Bengtsson, H. J., Kullberg, A., et al., “Serotonin autoreceptor function and antidepressant drug action,” J. Psychopharmacol., 14, No. 2, 177–185 (2000).
Holly, E. N. and Miczek, K. A., “Ventral tegmental area dopamine revisited: effects of acute and repeated stress,” Psychopharmacology (Berlin), 233, No. 2, 163–86 (2016).
Ilango, A., Kesner, A. J., Broker, C. J., et al., “Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses,” Front. Behav. Neurosci., 8, 155 (2014).
Ioannidis, J. P., “Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials?” Philos. Ethics Humanit. Med., 3, 14 (2008).
Kato, T., Kasahara, T., Kubota-Sakashita, M., et al., “Animal models of recurrent or bipolar depression,” Neuroscience, 321, 189–196 (2016).
Khan, A. and Brown, W. A., “Antidepressants versus placebo in major depression: an overview,” World Psychiatry, 14, 294–300 (2015).
Knowland, D. and Lim, B. K., “Circuit-based frameworks of depressive behaviors: The role of reward circuitry and beyond,” Pharmacol. Biochem. Behav. (2018), doi https://doi.org/10.1016/j.pbb.2017.12.010.
Knowland, D., Lilascharoen, V., Pacia, C. P., et al., “Distinct ventral pallidal neural populations mediate separate symptoms of depression,” Cell, 170, 1–14 (2017).
Koob, G. F., “The dopamine anhedonia hypothesis: a pharmacological phrenology,” Behav. Brain Sci., 5, 63–64 (1982).
Kudryavtseva, N. N. and Avgustinovich, D. F., “Molecular mechanisms of social behavior: commentary on the report by Berton et al.,” Neironauki, 4, No. 6, 33–35 (2006).
Kudryavtseva, N. N., “Serotoninergic control of aggressive behavior: new approaches – new interpretations (review),” Zh. Vyssh. Nerv. Deyat., 65, No. 5, 546–563 (2015).
Kudryavtseva, N. N., “The sensory contact model for the study of aggressive and submissive behaviors in male mice,” Aggress. Behav., 17, No. 5, 285–291 (1991).
Kudryavtseva, N. N., Bakshtanovskaya, I. V., and Koryakina, L. A., “Social model of depression in mice of C57BL/6J strain,” Pharmacol. Biochem. Behav., 38, 315–320 (1991).
Kudryavtseva, N. N., Smagin, D. A., Kovalenko, I. L., and Vishnivetskaya, G. B., “Repeated positive fighting experience in male inbred mice,” Nat. Protoc., 9, No. 11, 2705–2717 (2014).
Laurent, V., Leung, B., Maidment, N., Balleine, B. W., “μ- and δ-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice,” J. Neurosci., 32, No. 5, 1875–1883 (2012).
Laurent, V., Wong, F. L., and Balleine, B. W., “The lateral habenula and its input to the rostromedial tegmental nucleus mediates outcome-specific conditioned inhibition,” J. Neurosci., 37, No. 45, 10932–10942 (2017).
LeDoux, J. E. and Pine, D. S., “Using neuroscience to help understand fear and anxiety: a two-system framework,” Am. J. Psychiatry, 173, 1083–1093 (2016).
Li, B., Piriz, J., Mirrione, M., et al., “Synaptic potentiation onto habenula neurons in the learned helplessness model of depression,” Nature, 470, No. 7335, 535–539 (2011).
Lisman, J., Grace, A. A., and Duzel, E., “A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP,” Trends Neurosci., 34, No. 10, 536–547 (2011).
Liu, B., Liu, J., Wang, M., et al., “From serotonin to neuroplasticity: evolvement of theories for major depressive disorder,” Front. Cell. Neurosci., 11, 305, 1–9 (2017).
Liu, R., Jolas, T., and Aghajanian, G., “Serotonin 5HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus,” Brain Res., 873, 34–45 (2000).
Liu, R.-J., Ota, K. T., Dutheil, S., et al., “Ketamine strengthens CRFactivated amygdala inputs to basal dendrites in mPFC layer V pyramidal cells in the prelimbic but not infralimbic subregion, a key suppressor of stress responses,” Neuropsychopharmacology, 40, No. 9, 2066–2075 (2015).
Maier, S. F. and Seligman, M. E. P., “Learned helplessness at fifty: insights from neuroscience,” Psychol. Rev., 123, No. 4, 349–367 (2016).
Marcinkiewcz, C. A., Mazzone, C. M., D’Agostino, G., et al., “Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala,” Nature, 537, No. 7618, 97–101 (2016).
Mileykovskiy, B. and Morales, M., “Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear,” J. Neurosci., 31, No. 20, 7471–7476 (2011).
Miyazaki, K. W., Miyazaki, K., Tanaka, K. F., et al., “Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards,” Curr. Biol., 24, No. 17, 2033–2040 (2014).
Miyazaki, K., Miyazaki, K. W., and Doya, K., “Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards,” J. Neurosci., 31, No. 2, 469–479 (2011).
Miyazaki, K., Miyazaki, K. W., and Doya, K., “The role of serotonin in the regulation of patience and impulsivity,” Mol. Neurobiol., 45, 213–224 (2012).
Molendijk, M. L. and de Kloet, E. R., “Immobility in the forced swim test is adaptive and does not reflect depression,” Psychoneuroendocrinology, 62, 389–391 (2015).
Moscarello, J. M. and LeDoux, J. E., “Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions,” J. Neurosci., 33, No. 9, 3815–3823 (2013).
Mosolov, S. N., Anxiety and Depression: Comorbidity and Therapy, Artinfo Publishing, Moscow (2007).
Nakanishi, S., Hikida, T., and Yawata, S., “Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors,” Neuroscience, 282, 49–59 (2014).
Ohmura, Y., Tanaka, K. F., Tsunematsu, T., et al., “Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice,” Int. J. Neuropsychopharmacol., 17, 1777–1783 (2014).
Pavlova, I. V. and Rysakova, M. P., “Effects of administration of serotonin 5HT1A receptor ligands into the amygdala on the behavior of rats with different manifestations of conditioned reflex fear,” Zh. Vyssh. Nerv. Deyat., 66, No. 6, 710–724 (2016).
Pine, D. S. and LeDoux, J. E., “Elevating the role of subjective experience in the clinic: response to Fanselow and Pennington,” Am. J. Psychiatry, 174, No. 11, 1121–1122 (2017).
Robinson, S., Sandstrom, S. M., Denenberg, V. H., and Palmiter, R. D., “Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards,” Behav. Neurosci., 119, No. 1, 5–15 (2005).
Salamone, J. D., Yohn, S. E., López-Cruz, L., et al., “Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology,” Brain, 139, No. 5, 1325–1347 (2016).
Saul’skaya, N. B. and Marchuk, O. E., “Serotoninergic stimulation of the medial prefrontal cortex during acquisition of a conditioned reflex reaction to fear promotes the formation of generalized fear,” Zh. Vyssh. Nerv. Deyat., 68 (2018).
Schultz, W., Stauffer, W. R., and Lak, A., “The phasic dopamine signal maturing: from reward via behavioural activation to formal economic utility,” Curr. Opin. Neurobiol., 43, 139–148 (2017).
Selye, G., Stress without Distress, Moscow (1979).
Shenhav, A. and Botvinick, M. M., “Motivated action: new light on prefrontal-neuromodulatory circuits,” Curr. Biol., 23, No. 4, R161–R162 (2013).
Shiflett, M. W. and Balleine, B. W., “At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation,” Eur. J. Neurosci., 32, 1735–1743 (2010).
Slattery, D. A., Neumann, I. D., and Cryan, J. F., “Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat,” J. Psychopharmacol., 25, No. 10, 1295–1303 (2011).
Srejic, L. R., Hamani, C., and Hutchison, W. D., “High-frquency stimulation of the medial prefrontal cortex decreases cellular firing in the dorsal raphe,” Eur. J. Neurosci., 41, No. 9, 1219–26 (2015).
Teissier, A., Chemiakine, A., Inbar, B., et al., “Activity of raphe serotonergic neurons controls emotional behaviors,” Cell Rep., 13, No. 9, 1965–1976 (2015).
Thomson, A. A. and Martinet, A. V., A Practical English Grammar, Oxford University Press (1986).
Treadway, M. T. and Zald, D. H., “Reconsidering anhedonia in depression: lessons from translational neuroscience,” Neurosci. Biobehav. Rev., 35, No. 3, 537–555 (2011).
Treadway, M. T., “The Neurobiology of motivational deficits in depression – an update on candidate pathomechanisms,” Curr. Top. Behav. Neurosci., 27, 337–55 (2015).
Tye, K. M., Mirzabekov, J. J., Warden, M. R., et al., “Dopamine neurons modulate neural encoding and expression of depression-related behaviour,” Nature, 493, No. 7433, 537–541 (2013).
Urban, D. J., Zhu, H., Marcinkiewcz, C. A., et al., “Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons,” Neuropsychopharmacology, 41, No. 5, 1404–15 (2016).
Veerakumar, A., Challis, C., Gupta, P., et al., “Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems,” Biol. Psychiatry, 76, No. 3, 203– 212 (2014).
Warden, M. R., Selimbeyoglu, A., Mirzabekov, J. J., et al., “A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge,” Nature, 492, No. 7429, 428–432 (2012).
Wassum, K. M., Ostlund, S. B., Maidment, N. T., and Balleine, B. W., “Distinct opioid circuits determine the palatability and the desirability of rewarding events,” Proc. Natl. Acad. Sci. USA, 106, No. 30, 12512–12517 (2009).
Weissbourd, B., Ren, J., DeLoach, K. E., et al., “Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons,” Neuron, 83, No. 3, 645–662 (2014).
Wenzel, J. M., Oleson, E. B., Gove, W. N., et al., “Phasic dopamine signals in the nucleus accumbens that cause active avoidance require endocannabinoid mobilization in the midbrain,” Curr. Biol., 28, 1–13 (2018).
Wenzel, J. M., Rauscher, N. A., Cheer, J. F., and Oleson, E. B., “A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature,” ACS Chem. Neurosci., 6, No. 1, 16–26 (2015).
Wise, R. A., “Dual roles of dopamine in food and drug seeking: the drive-reward paradox,” Biol. Psychiatry, 73, No. 9, 819–826 (2013).
Xiao, J., Song, M., Li, F., et al., “Effects of GABA microinjection into dorsal raphe nucleus on behavior and activity of lateral habenular neurons in mice,” Exp. Neurol., 298, 23–30 (2017).
Xu, S., Das, G., Hueske, E., and Tonegawa, S., “Dorsal raphe serotonergic neurons control intertemporal choice under trade-off,” Curr. Biol., 27, 1–9 (2017).
Yadid, G. and Friedman, A., “Dynamics of the dopaminergic system as a key component to the understanding of depression,” Prog. Brain Res., 172, 265–286 (2008).
Yagishita, S., Hayashi-Takagi, A., Ellis-Davies, G. S. R., et al., “A critical time window for dopamine actions on the structural plasticity of dendritic spines,” Science, 345, No. 6204, 1616–1620 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 68, No. 4, pp. 415–428,
July–August, 2018.
Rights and permissions
About this article
Cite this article
Latanov, A.V., Korshunov, V.A., Maiorov, V.I. et al. Serotonin and Dopamine in Biological Models of Depression. Neurosci Behav Physi 49, 987–995 (2019). https://doi.org/10.1007/s11055-019-00828-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00828-7