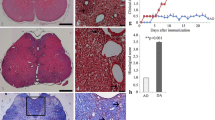
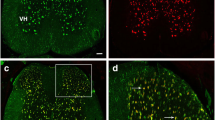
The limited regenerative capacity of the central nervous system (CNS) is one of the key factors promoting the development of neurodegenerative diseases, including multiple sclerosis. The regenerative potential of CNS cells can be evaluated in terms of the level of GAP-43 protein, which is involved in the processes of neuritogenesis and growth cone navigation. Cleavage of GAP-43 by m-calpain to form the fragment GAP-43-3, which lacks 40 N-terminal amino acids, probably leads to loss of the initial functions of the protein, which may have adverse influences on the regenerative potential of the CNS. The present study was performed on the most appropriate model of multiple sclerosis – experimental allergic encephalomyelitis (EAE) in rats. The spinal cord of animals with EAE was shown to have inflammatory infiltrates and deformed motoneurons; limb pareses and paralysis developed. When EAE was mild, about 60% of GAP-43 in rat spinal cord cells was present in the form of GAP-43-3. In severe forms of EAE, with tetraplegia, the extent of GAP-43 proteolysis by calpain reached 85%. In this situation, GAP-43-3 was found mainly in microglial cells. We suggest that during the development of EAE, proteolytic cleavage of GAP-43 protein by m-calpain to form GAP-43-3 can significantly alter its properties, suppressing the regenerative ability of CNS cells and exacerbating the course of the pathological process.
Similar content being viewed by others
References
D. E. Korzhevskii, O. V. Kirik, and M. N. Karpenko, Theoretical Grounds and Practical Applications of Immunohistochemical Methods: A Handbook, SpetsLit, St. Petersburg (2012).
K. R. Bulsara, B. J. Iskandar, A. T. Villavicencio, and J. H. Skene, “A new millennium for spinal cord regeneration: growth-associated genes,” Spine, 27, No. 17, 1946–1949 (2002).
L. C. Case and M. Tessier-Lavigne, “Regeneration of the adult central nervous system,” Curr. Biol., 15, No. 18, 749–753 (2005).
M. S. Chong, C. J. Woolf, M. Turmaine, et al., “Intrinsic versus extrinsic factors in determining the regeneration of the central processes of rat dorsal root ganglion neurons: the influence of a peripheral nerve graft,” J. Comp. Neurol., 370, No. 1, 97–104 (1996).
P. J. Coggins and H. Zwiers, “Evidence for a single protein kinase C-mediated phosphorylation site in rat brain protein B-50,” J. Neurochem., 53, No. 6, 1895–1901 (1989).
M. Dong, R. Liu, L. Guo, et al., “Pathological findings in rats with experimental allergic encephalomyelitis,” APMIS, 116, No. 11, 972–984 (2008).
E. G. Gilerovich, E. A. Fedorova, L. N. Abdurasulova, et al., “Analysis of the morphological signs of an inflammatory reaction in the spinal cord of Wistar rats in an experimental model,” Neurosci. Behav. Physiol., 42, No. 1, 43–47 (2012).
Q. He, E. Dent, and K. F. Meiri, “Modulation of actin fi lament behavior by GAP-43 (neuromodulin) is dependent on the phosphoryla-tion status of serine 41, the protein kinase C site,” J. Neurosci., 17, No. 10, 3515–3524 (1997).
M. N. Karpenko, I. N. Abdulrasulova, and V. M. Klimenko, “Calpain activity in spinal cord cells from rats with experimental allergic encephalomyelitis of different grades,” Neurosci. Behav. Physiol., 41, No. 3, 252–258 (2011).
T. Kawasaki, T. Nishio, S. Kawaguchi, and H. Kurosawa, “Spatiotemporal distribution of GAP-43 in the developing rat spinal cord: a histological and quantitative immunofluorescence study,” Neurosci. Res., 39, No. 3, 347–358 (2001).
M. Kerschensteiner, F. M. Bareyre, B. S. Buddeberg, et al., “Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis,” J. Exp. Med., 200, No. 8, 1027–1038 (2004).
P. J. Meberg, C. M. Gall, and A. Routtenberg, “Induction of F1/GAP-43 gene expression in hippocampal granule cells after seizures,” Brain Res. Mol. Brain Res., 17, No. 3–4, 295–299 (1993).
M. I. Mosevitsky, V. A. Notitskaya, A. Yu. Plekhanov, and G. Yu. Skladchikova, “Neuronal “protein GAP-43 is a member of novel group of brain acid-soluble proteins (BASPs),” Neurosci. Res., 19, No. 2, 223– 228 (1994).
A. Nicot, P. V. Ratnakar, Y. Ron, et al., “Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction,” Brain, 126, No. 2, 398–412 (2003).
A. B. Oestreicher, P. N. De Graan, W. H. Gispen, et al., “B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system,” Prog. Neurobiol., 53, No. 6, 627–686 (1997).
S. Panyim and R. Chalkley, “High-resolution acrylamide gel electrophoresis of histones,” Arch. Biochem. Biophys., 130, No. 1, 337–346 (1969).
M. Penkowa and J. Hidalgo, “Treatment with metallothionein prevents demyelination and axonal damage and increases oligodendrocyte precursors and tissue repair during experimental autoimmune encephalomyelitis,” J. Neurosci. Res., 72, No. 5, 574–586 (2003).
A. Y. Plekhanov, “Immunoreplica from the gel surface: rapid and sensitive blot plus intact gel,” Anal. Biochem., 239, No. 1, 110–111 (1996).
M. Sensenbrenner, M. Lucas, and J. C. Deloulm, “Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells,” J. Mol. Med., 75, No. 9, 653–663 (1997).
P. Singh, P. K. Heera, and G. Kaur, “Expression of neuronal plasticity markers in hypoglycemia-induced brain injury,” Mol. Cell. Biochem., 247, No. 1–2, 69–74 (2003).
C. E. Teunissen, C. D. Dijkstra, B. Jasperse, et al., “Growth-associated protein 43 in lesions and cerebrospinal fluid in multiple sclerosis,” Neuropathol. Appl. Neurobiol., 32, No. 3, 318–331 (2006).
P. S. Vosler, C. S. Brennan, and J. Chen, “Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration,” Mol. Neurobiol., 38, No. 1, 78–100 (2008).
V. V. Zakharov, M. N. Bogdanova, and M. I. Mosevitsky, “Specific proteolysis of neuronal protein GAP-43 by calpain: characterization, regulation, and physiological role,” Biochemistry (Moscow), 70, No. 8, 897–890 (2005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 101, No. 1, pp. 74–84, January, 2015.
Rights and permissions
About this article
Cite this article
Tikhomirova, M.S., Karpenko, M.N., Kirik, O.V. et al. GAP-43 Protein and Its Proteolytic Fragment in Spinal Cord Cells in Rats with Experimental Allergic Encephalomyelitis. Neurosci Behav Physi 46, 582–588 (2016). https://doi.org/10.1007/s11055-016-0282-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-016-0282-1