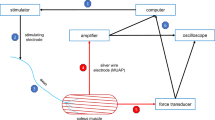
Developmental changes in the contractile and electrical responses of isolated fast (tibialis anterior) and slow (soleus) muscles from chick embryo (16–20 days of embryonic development) were studied, as were the effects of hypoxia on them. Normalized contractile response forces for tibialis anterior were significantly greater than those for soleus. On days 16–17 of embryogenesis, soleus and tibialis anterior muscle fibers showed slow decaying oscillation waves of excitation, while extracellular recording identified propagating action potentials in 20% of the tibialis anterior muscle fibers studied. By day 20 of embryonic development, the number of muscle fibers able to generate action potentials in fast muscles approached 100%. In slow muscles, this value was close to 50%. Hypoxia induced a reduction in the strength of the contractile responses of muscles at all study time points during the incubatory development of embryos, but was not found to have any effect on the magnitude of the contractile response evoked by caffeine. These results lead to the conclusion that in these conditions, hypoxia has no influence on the functional state of ryanodine receptors. Muscles treated with insulin and ouabain showed significant reductions in the sensitivity of contractile responses to hypoxia. It is suggested that the effects of hypoxia involve an active role for membrane Na+,K+-ATPase.
Similar content being viewed by others
References
I. V. Kubasov, R. S. Arutyunyan, M. G. Dobretsov, and E. V. Matrosova, “The action of insulin on the contractile and electrical responses of rat skeletal muscle,” Ros. Fiziol. Zh., 9, No. 10, 1200–1213 (2012).
I. V. Kubasov, M. V. Nechaeva, T. A. Alekseeva, et al., “Characteristics of the contractile responses of isolated fast and slow chick embryo muscles on changes in the solution oxygen level,” in: New Approaches to the Study of Classical Problems, MGU, Moscow (2013).
G. A. Nasledov, I. E. Katina, and Yu. V. Zhitnikova, “Characteristics of the functioning of the electromechanical linkage in striate muscle in higher and lower vertebrates,” Biofi zika, 47, No. 4, 716–728 (2002).
B. T. Ameredes, M. W. Julian, and T. L. Clanton, “Muscle shortening increases sensitivity of fatigue to severe hypoxia in canine diaphragm,” J. Appl. Physiological., 71, No. 6, 2309–2316 (1991).
A. Bekoff, “Neuroethological approaches to the study of motor development in chicks: achievements and challenges,” J. Neurobiol., 23, No. 10, 1486–1505 (1992).
N. S. Bradley, “Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility,” J. Neurophysiol., 86, No. 4, 1511–1522 (2001).
M. A. Brotto, S. Andreatta-ven Leyen, C. M. Nosek, et al., “Hypoxia and fatigue-induced modification of function and proteins in intact and skinned murine diaphragm muscle,” Pflugers Arch.-Eur. J. Physiol., 440, No. 5, 727–734 (2000).
S. P. Cornfield, “The mechanical properties and heat production of chicken latissimus dorsi muscles during tetanic contractions,” J. Physiol., 219, No. 2, 281–302 (1971).
T. Clausen, “Na+K+ pump regulation and skeletal muscle contractility,” Physiol. Rev., 83, 1269–1324 (2003).
S. A. Esau, “Hypoxic, hypercapnic acidosis decreases tension and increases fatigue in hamster diaphragm muscle in vitro,” Am. Rev. Respir. Dis., 139, No. 6, 1410–1417 (1989).
V. Hamburger and M. Balaban, “Observation and experiments on spontaneous rhythmical behavior in the chick embryo,” Dev. Biol., 7, 533–545 (1963).
R. I. Hume and S. A. Thomas, “A calcium and voltage-dependent chloride current in developing chick skeletal muscle,” J. Physiol., 417, 241–261 (1989).
I. V. Kubasov and M. Dobretsov, “Two types of extracellular action potentials recorded with narrow-tipped pipettes in skeletal muscle of frog, Rana temporaria,” J. Physiol., 590, No. 12, 937–944 (2012).
W. Melzer, A. Herrman-Frank, and H. C. Luttgau, “The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres,” Biochem. Biophys. Acta, 1241, No. 1, 59–116 (1995).
J. P. Mortola, A. S. Louis, M. Simeonova, and P. A. Toro Velazquez, “The motility of the chicken embryo: Energetic cost and effects of hypoxia,” Respirat. Physiol. Neurobiol., 188, No. 2, 172–179 (2013).
G. B. Müller, “Embryonic motility: environmental influences and evolutionary innovation,” Evol. Dev., 5, No. 1, 56–60 (2003).
M. V. Nechaeva, I. G. Vladimirova, and T. A. Alexeeva, “Effect of acute hypoxia on the motor activity and heart rate of the 10- and 14-day chick embryo,” Open Ornithol. J., 3, 127–133 (2010).
G. S. Posterino, M. A. Cellini, and G. D. Lamb, “Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle,” J. Physiol., 547, No. 3, 807–823 (2003).
R. J. Salomone and R. Van Lunteren, “Effects of hypoxia and hypercapnia on geniohyoid contractility and endurance,” J. Appl. Physiol., 71, No. 2, 709–715 (1991).
D. G. Stephenson, “Tubular system excitability: an essential component of excitation-contraction coupling in fast-twitch fibres of vertebrate skeletal muscle,” J. Muscle Res. Cell Motil., 27, No. 5, 259–274 (2006).
E. Van Lunteren, M. Moyer, and A. Torres, “ATP-sensitive K channel blocker glibenclamide and diaphragm fatigue during normoxia and hypoxia,” J. Appl. Physiol., 85, No. 2, 601–608 (1998).
E. Van Lunteren, A. Torres, and M. Moyer, “Effects of hypoxia on diaphragm relaxation rate during fatigue,” J. Appl. Physiol., 82, No. 5, 1472–1478 (1997).
J. M. West, C. J. Barclay, A. R. Luff, and D. W. Walker, “Developmental changes in the activation properties and ultrastructure of fast- and slow-twitch muscles from fetal sheep,” J. Muscle Res. Cell Motil., 20, No. 3, 249–264 (1999).
H. Wolters, W. Wallinga, D. L. Ypey, and H. B. K. Boom, “Ionic current during action potential in mammalian skeletal muscle fibers analyzed with loose patch clamp,” Am. J. Physiol. Cell. Physiol., 267, No. 6, 1699–1706 (1994).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 100, No. 2, pp. 187–200, February, 2014.
Rights and permissions
About this article
Cite this article
Kubasov, I.V., Nechaeva, M.V. & Alekseeva, T.A. Effects of Hypoxia on the Contractile and Electrical Responses of Chick Embryo Skeletal Muscles in the Last Third of Embryogenesis. Neurosci Behav Physi 45, 894–901 (2015). https://doi.org/10.1007/s11055-015-0163-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-015-0163-z