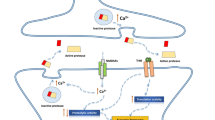
The Wnt signal pathway is a signal mechanism in which the key transmitter molecules are peptides of the Wnt family. This review analyzes data on the involvement of this transmembrane signal cascade (canonical and noncanonical) in structural and synaptic plasticity processes in the hippocampus. The mechanisms of the involvement of the Wnt signal pathway in the normal functioning of neuroplasticity is discussed, as are impairments to these mechanisms underlying cerebral pathologies. Particular attention is paid to what is from the points of view of plasticity and neuropathology one of the most important components of the canonical Wnt pathway – the enzyme glycogen synthase kinase (GSK-3β). Studies in this area have fundamental value as well as significant potential for translation, and the key components of the Wnt pathway are potential targets for the development of pathogenetically based treatments for socially important neurological and mental diseases.
Similar content being viewed by others
References
M. E. Avila, F. J. Sepulveda, C. F. Burgos, et al., “Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons,” J. Biol. Chem., 285, No. 24, 18,939–18,947 (2010).
V. Beaumont, S. A. Thompson, F. Choudhry, et al., “Evidence for an enhancement of excitatory transmission in adult CNS by Wnt signaling pathway modulation,” Mol. Cell. Neurosci., 35, No. 4, 513–524 (2007).
F. Cai, F. Wang, F. K. Lin, et al., “Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway,” Free Radic. Biol. Med., 45, No. 7, 964–970 (2008).
W. Cerpa, A. Gambrill, N. C. Inestrosa, and A. Barria, “Regulation of NMDA-receptor synaptic transmission by Wnt signaling,” J. Neurosci., 31, No. 26, 9466–9471 (2011).
J. Chen, C. S. Park, and S. J. Tang, “Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation,” J. Biol. Chem., 281, No. 17, 11,910–11,916 (2006).
L. Conboy, C. M. Seymour, M. P. Monopoli, et al., “Notch signaling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats,” Neurobiol. Learn. Mem., 88, No. 3, 342–351 (2007).
I. Dewachter, L. Ris, S. Croes, et al., “Modulation of synaptic plasticity and Tau phosphorylation by wild-type and mutant presenilin1,” Neurobiol. Aging, 29, No. 5, 639–652 (2008).
J. Du, Y. Wei, L. Liu, et al., “A kinesin signaling complex mediates ability of GSK-3beta to affect mood-associated behaviors,” Proc. Natl. Acad. Sci. USA, 107, No. 25, 11,573–11,578 (2010).
C. Fuerer and R. Nusse, “Lentiviral vectors to prove and manipulate the Wnt signaling pathway,” PLoS One, 5, No. 2, e9370 (2010).
K. P. Giese, “GSK-3: a key player in neurodegeneration and memory,” IUBMB Life, 61, No. 5, 516–521 (2009).
I. A. Graef, P. G. Mermelstein, K. Stankunas, et al., “L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons,” Nature, 401, No. 6754, 703–708 (1999).
M. Gómez Ravetti, O. A. Rosso, R. Berretta, and P. Moscato, “Uncovering molecular biomarkers that correlate cognitive decline with the changes of hippocampus’ gene expression profiles in Alzheimer’s disease,” PLoS One, 5, No. 4, e10153 (2010).
J. G. Hong, D. H. Kim, C. H. Lee, et al., “GSK-3β activity in the hippocampus is required for memory retrieval,” Neurobiol. Learn. Mem., 98, No. 2, 122–129 (2012).
C. Hooper, V. Markevich, F. Plattner, et al., “Glycogen synthase kinase-3 inhibition is integral to long-term potentiation,” Eur. J. Neurosci., 25, No. 1, 81–86 (2007).
S. Jessberger, R. E. Clark, N. J. Broadbent, et al., “Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats,” Learn. Mem., 16, No. 2, 147–154 (2009).
S. Jimenez, M. Torres, M. Vizuete, et al., “Age-dependent accumulation of soluble amyloid beta (Abeta) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha [sApp(alpha)] by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer’s mouse model,” J. Biol. Chem., 286, No. 21, 18,414–18,425 (2011).
U. G. Kang, M. S. Roh, J. R. Jung, et al., “Activation of protein kinase B (Akt) signaling after electroconvulsive shock in the rat hippocampus,” Prog. Neuropsychopharmacology Biol. Psychiatry, 28, No. 1, 41–44 (2004).
D. U. Kumar and H. Devaraj, “Expression of Wnt3a, β-catenin, cyclin D1, and PCNA in mouse dentate gyrus subgranular zone (SGZ): a possible role of Wnt pathway in SGZ neural stem cell proliferation,” Folia Biol. (Praha), 58, No. 3, 115–120 (2012).
S. Li, S. Hong, N. E. Shepardson, D. M. Walsh, et al., “Soluble oligomers of amyloid beta protein facilitate long-term depression by disrupting neuronal glutamate uptake,” Neuron, 62, No. 6, 788–801 (2009).
T. Ma, N. Tzavaras, P. Tsokas, et al., “Synaptic stimulation of mTOR is mediated by Wnt signaling and regulation of glycogen synthase,” J. Neurosci., 31, No. 48, 17,537–17,546 (2011).
T. Miyaoka, H. Seno, and H. Ishino, “Increased expression of Wnt-1 in schizophrenic brains,” Schizophr. Res., 38, No. 1, 1–6 (1999).
D. C. Morris, Z. G. Zhang, Y. Wang, et al., “Wnt expression in the adult rat subventricular zone after stroke,” Neurosci. Lett., 418, No. 2, 170–174 (2007).
D. H. Oh, Y. C. Park, and S. H. Kim, “Increased glycogen synthase kinase-3β mRNA level in the hippocampus of patients with major depression: a study using the Stanley neuropathology consortium integrative database,” Psychiatry Investig., 7, No. 3, 202–207 (2010).
S. Peineau, S. C. Nicolas, Z. A. Bortolotto, et al., “A systemic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine-threonine kinases,” Mol. Brain, 2, 22 (2009).
J. Prickaerts, D. Moechars, K. Cryns, et al., “Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania,” J. Neurosci., 26, No. 35, 9022–9029 (2006).
W. B. Rowe, E. M. Blalock, K. C. Chen, et al., “Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats,” J. Neurosci., 27, No. 12, 3098–3110 (2007).
M. Sieber-Blum, “Ontogeny and plasticity of adult hippocampal neural stem cells,” Dev. Neurosci., 25, No. 2–4, 273–278 (2003).
A. Shruster, T. Ben-Zur, E. Melamed, and D. Offen, “Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury,” PLoS One, 7, No. 7, e40843 (2012).
R. Silva, A. R. Mesquita, J. Nessa, et al., “Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta,” Neuroscience, 152, No. 3, 656–669 (2008).
A. M. Stranahan, K. Lee, K. G. Becker, et al., “Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice,” Neurobiol. Aging, 31, No. 11, 1937–1949 (2010).
A. Szamosi, O. Kelemen, and S. Kéri, “Hippocampal volume and the AKT signaling system in first-episode schizophrenia,” J. Psychiatr. Res., 46, No. 3, 279–284 (2012).
N. Tabatadze, C. Tomas, R. McGonigal, et al., “Wnt transmembrane signaling and long-term spatial memory,” Hippocampus, 22, No. 6, 1228–1241 (2012).
L. Varela-Nallar, V. T. Ramirez, C. Gonzalez-Billault, and N. C. Inestrosa, “Frizzled receptors in neurons: From growth cones to the synapse,” Cytoskeleton (Hoboken), 69, No. 7, 528–534 (2012).
L. Varela-Nallar, I. E. Alfaro, F. G. Serrano, et al., “Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses,” Proc. Natl. Acad. Sci. USA, 107, No. 49, 21,164–21,169 (2010).
L. Varela-Nallar, C. P. Gabowsi, I. E. Alfaro, et al., “Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function,” Neural Dev., 4, 41 (2009).
L. C. Wei, Y. X. Ding, Y. H. Liu, et al., “Low-dose radiation stimulates Wnt/β-catenin signaling, neural stem cell proliferation and neurogenesis of the mouse hippocampus in vitro and in vivo,” Curr. Alzheimer Res., 9, No. 3, 278–289 (2012).
Y. F. Xie, J. C. Belrose, G. Lei, et al., “Dependence of NMDA/GSK-3β mediated plasticity on TRPM2 channels at hippocampal CA3-CA1 synapses,” Mol. Brain, 4, 44 (2011).
X. Xu, M. Zhan, W. Dan, et al., “Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender,” Genome Biol., 8, No. 11, R234 (2007).
R. Zhou, P. Yuan, Y. Wang, et al., “Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers,” Neuropsychopharmacology, 34, No. 6, 1395–1405 (2009).
L. Q. Zhu, S. H. Wang, D. Liu, et al., “Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments,” J. Neurosci., 27, No. 45, 12,211–12,220 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 98, No. 12, pp. 1460–1470, December, 2012.
Rights and permissions
About this article
Cite this article
Markevich, V.A., Salozhin, S.V. & Gulyaeva, N.V. Involvement of the Wnt Signal Pathway in Hippocampal Plasticity. Neurosci Behav Physi 44, 810–816 (2014). https://doi.org/10.1007/s11055-014-9988-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-9988-0