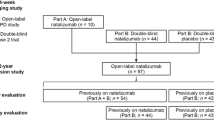
The “Sovet” post-marketing observational program was started in 2011. A total of 69 patients with the remitting form of multiple sclerosis (MS) treated with the second-generation DMD natalizumab (NZ) were studied. NZ infusions were given over 1 h every four weeks for 12 months. Treatment produced significant reductions in the frequency of exacerbations of disease, from 2.22 ± 0.98 to 0.18 ± 0.42 per year, along with a tendency to decreased EDSS scores, from 3.69 ± 1.0 to 3.37 ± 1.17. Adverse events occurred during treatment in 11 patients, each reaction being seen in no more than one person; treatment withdrawal was required in only one case, because of a generalized allergic reaction. The data presented here supplement published data on the positive effects of NZ on the course and signs of MS. These results lead to the conclusion that strict selection of patients and correct management during NZ treatment can provide a positive balance between the benefits of this treatment and its potential risks.
Similar content being viewed by others
References
Biogen Idec, Natalizumab Safety Review [Russian translation] (2012).
C. Bozic, L. M. Cristiano, A. Natarajan, et al., “Utilisation and safety of natalizumab in patients with relapsing multiple sclerosis,” Multiple Sclerosis, 16, 494 (2010).
P. K. Coyle, J. F. Foley, E. J. Fox, et al., “Best practice recommendations for the selection and management of patients with MS receiving natalizumab therapy,” Multiple Sclerosis, 15, 26–36 (2009).
A. Egli, L. Infanti, A. Dumoulin, et al., “Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors,” J. Infect. Dis., 199, 837–846 (2009).
E. Havrdova, S. Galetta, M. Hutchinson, et al., “Effects of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the natalizumab safety and efficacy in relapsing-remitting multiple sclerosis “AFFIRM” study,” Lancet Neurol., 8, No. 3, 254–260 (2009).
L. Kappos, D. Bates, P. Hartung, et al., “Natalizumab treatment for multiple sclerosis: updated recommendations for patient monitoring and selection,” Lancet Neurol., 10, 745–758 (2011).
J. M. Kean, S. Rao, M. Wang, and R. L. Garcea, “Seroepidemiology of human polyomaviruses,” PLoS Pathol., 5, No. 3, 1000363 (2009).
W. A. Knowles, P. Pipkin, N. Andrews, et al., “Population-based study of antibody of the human polyomaviruses BKV and JCV and the similar polyomavirus SV40,” J. Med. Virol., 71, No. 1, 115–123 (2003).
F. Munschauer, G. Giovannoni, F. Lublin, et al., “Natalizumab significantly increases the cumulative probability of sustained improvement in physical disability,” Presentation at WCTRIMS, Montreal (2008).
E. Pucci, G. Giuliani, A. Solari, et al., “Natalizumab for relapsing remitting multiple sclerosis,” Cochrane Database Syst. Rev., Iss. 10 (2011).
C. Tornatore and D. B. Clifford, “Clinical vigilance for progressive multifocal leukoencephalopathy in the context of natalizumab use,” Multiple Sclerosis, 15, 16–25 (2009).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The principal investigators in the “Sovet” study program and the members of the coordinating committee are: A. N. Boiko, Ya. V. Vlasov, I. A. Zavalishin, M. N. Zakharova, E. P. Evdoshenko, S. V. Kotov, L. I. Volkova, N. A. Malkova, A. S. Fedyanin, Yu. V. Trinitatskii, K. Z. Bakhtiyarova, L. A. Tsukurova, G. N. Bel'skaya, E. I. Ivashinenkova, A. A. Gavrilenko, T. A. Shcherbanosova, A. S. Rozhdestvenskii, N. N. Spirin, and I. O. Stepanov.
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 113, No. 2, Iss. II, Multiple Sclerosis, pp. 79–82, February 2013.
Rights and permissions
About this article
Cite this article
Popova, E.V., Brylev, L.V., Davydovskaya, M.V. et al. Preliminary Results of the “Sovet” Observational Program for Use of Natalizumab in the Treatment of Patients with Remitting Multiple Sclerosis. Neurosci Behav Physi 44, 520–523 (2014). https://doi.org/10.1007/s11055-014-9943-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-9943-0