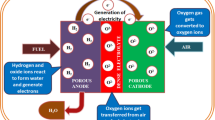
Abstract
Platinum-loaded carbon is commonly used in polymer electrolyte fuel cells (PEFCs); however, it is known to corrode or degrade under high potentials, which results in poor cell performance. Niobium-doped tin oxide (Nb-SnO2; NTO) nanoparticles are alternative materials to carbon because of their high durability and good cell performance as catalyst support in fuel cells. Here, we introduce the preparation of NTO nanoparticles with high specific surface area by spray flames. The particle characteristics and PEFC performances of the nanoparticles were evaluated. Spray combustion with a two-fluid nozzle was used where the raw material species were rapidly gasified to form fine nanoparticles. The spray flame synthesis was operated at a combustion enthalpy density of 4.87 kJ/ggas. This enabled homogeneous nanoparticle formation and suppressed particle growth under a minimal condition. The flame-made NTO nanoparticles showed a primary particle size and specific surface area of ~ 8.77 nm and 87.04 m2/g, respectively. Rietveld analysis revealed a detailed crystal structure of the NTO nanoparticles. In addition, Pt was loaded on the NTO nanoparticles and the cell performance of the resulting material was assessed using a membrane electrode assembly. The results of this study can be used to improve the features of flame-made NTO nanoparticles in order to suit the needs of a fuel cell application.






Similar content being viewed by others
References
Kodama K, Nagai T, Kuwaki A, Jinnouchi R, Morimoto Y (2021) Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat Nanotechnol 16:140–147. https://doi.org/10.1038/s41565-020-00824-w
Jiao K, Xuan J, Du Q, Bao Z, Xie B, Wang B, Zhao Y, Fan L, Wang H, Hou Z, Huo S, Brandon NP, Yin Y, Guiver MD (2021) Designing the next generation of proton-exchange membrane fuel cells. Nature 595:361–369. https://doi.org/10.1038/s41586-021-03482-7
Staffell I, Scamman D, Velazquez Abad A, Balcombe P, Dodds PE, Ekins P, Shah N, Ward KR (2019) The role of hydrogen and fuel cells in the global energy system. Energy Environ Sci 12:463–491. https://doi.org/10.1039/c8ee01157e
Zhang J, Yuan Y, Gao L, Zeng G, Li M, Huang H (2021) Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: fundamental understanding and design strategies. Adv Mater 33:e2006494. https://doi.org/10.1002/adma.202006494
Suter TAM, Smith K, Hack J, Rasha L, Rana Z, Angel GMA, Shearing PR, Miller TS, Brett DJL (2021) Engineering catalyst layers for next-generation polymer electrolyte fuel cells: a review of design, materials, and methods. Adv Energy Mater 11:2101025. https://doi.org/10.1002/aenm.202101025
Cullen DA, Neyerlin KC, Ahluwalia RK, Mukundan R, More KL, Borup RL, Weber AZ, Myers DJ, Kusoglu A (2021) New roads and challenges for fuel cells in heavy-duty transportation. Nat Energy 6:462–474. https://doi.org/10.1038/s41560-021-00775-z
Zhang Z, Liu J, Gu J, Su L, Cheng L (2014) An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ Sci 7:2535–2558. https://doi.org/10.1039/c3ee43886d
Takasaki F, Matsuie S, Takabatake Y, Noda Z, Hayashi A, Shiratori Y, Ito K, Sasaki K (2011) Carbon-free Pt electrocatalysts supported on SnO2 for polymer electrolyte fuel cells: electrocatalytic activity and durability. J Electrochem Soc 158:B1270–B1275. https://doi.org/10.1149/1.3625918
Kakinuma K, Suda K, Kobayashi R, Tano T, Arata C, Amemiya I, Watanabe S, Matsumoto M, Imai H, Iiyama A, Uchida M (2019) Electronic states and transport phenomena of Pt nanoparticle catalysts supported on Nb-doped SnO2 for polymer electrolyte fuel cells. ACS Appl Mater Interfaces 11:34957–34963. https://doi.org/10.1021/acsami.9b11119
Shi G, Tano T, Tryk DA, Iiyama A, Uchida M, Kakinuma K (2021) Temperature dependence of oxygen reduction activity at Pt/Nb-doped SnO2 catalysts with varied Pt loading. ACS Catal 11:5222–5230. https://doi.org/10.1021/acscatal.0c05157
Matsumoto S, Nagamine M, Noda Z, Matsuda J, Lyth SM, Hayashi A, Sasaki K (2018) PEFC Electrocatalysts supported on Nb-SnO2 for MEAs with high activity and durability: part II. Application of bimetallic Pt-alloy catalysts. J Electrochem Soc 165:F1164–F1175. https://doi.org/10.1149/2.0321814jes
Takei C, Kobayashi R, Mizushita Y, Hiramitsu Y, Kakinuma K, Uchida M (2018) Platinum anti-dissolution mechanism of Pt/Nb-SnO2 cathode catalyst layer during load cycling in the presence of oxygen for polymer electrolyte fuel cells. J Electrochem Soc 165:F1300–F1311. https://doi.org/10.1149/2.0591816jes
Senoo Y, Kakinuma K, Uchida M, Uchida H, Deki S, Watanabe M (2014) Improvements in electrical and electrochemical properties of Nb-doped SnO2-δ supports for fuel cell cathodes due to aggregation and Pt loading. RSC Adv 4:32180–32188. https://doi.org/10.1039/c4ra03988b
Kakinuma K, Chino Y, Senoo Y, Uchida M, Kamino T, Uchida H, Deki S, Watanabe M (2013) Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2-δ support materials with aggregated structure by rotating disk electrode and fuel cell measurements. Electrochim Acta 110:316–324. https://doi.org/10.1016/j.electacta.2013.06.127
Hirano T, Tsuboi T, Tanabe E, Ogi T (2022) In-situ flame deposition of Pt catalysts on Nb-doped SnO2 nanoparticles. J Alloys Compd 898:162749. https://doi.org/10.1016/j.jallcom.2021.162749
Li S, Ren Y, Biswas P, Tse SD (2016) Flame aerosol synthesis of nanostructured materials and functional devices: processing, modeling, and diagnostics. Prog Energy Combust Sci 55:1–59. https://doi.org/10.1016/j.pecs.2016.04.002
Teoh WY, Amal R, Madler L (2010) Flame spray pyrolysis: an enabling technology for nanoparticles design and fabrication. Nanoscale 2:1324–1347. https://doi.org/10.1039/c0nr00017e
Strobel R, Pratsinis SE (2007) Flame aerosol synthesis of smart nanostructured materials. J Mater Chem 17:4743–4756. https://doi.org/10.1039/b711652g
Pokhrel S, Madler L (2020) Flame-made particles for sensors, catalysis, and energy storage applications. Energy Fuels 34:13209–13224. https://doi.org/10.1021/acs.energyfuels.0c02220
Kakinuma K, Uchida M, Kamino T, Uchida H, Watanabe M (2011) Synthesis and electrochemical characterization of Pt catalyst supported on Sn0.96Sb0.04O2−δ with a network structure. Electrochim Acta 56:2881–2887. https://doi.org/10.1016/j.electacta.2010.12.077
Chino Y, Kakinuma K, Tryk DA, Watanabe M, Uchida M (2015) Influence of Pt loading and cell potential on the HF ohmic resistance of an Nb-Doped SnO2-supported Pt cathode for PEFCs. J Electrochem Soc 163:F97–F105. https://doi.org/10.1149/2.0571602jes
Kakinuma K, Kobayashi R, Iiyama A, Uchida M (2018) Influence of ionomer content on both cell performance and load cycle durability for polymer electrolyte fuel cells using Pt/Nb-SnO2 cathode catalyst layers. J Electrochem Soc 165:J3083–J3089. https://doi.org/10.1149/2.0141815jes
Wang W-N, Purwanto A, Lenggoro IW, Okuyama K, Chang H, Jang HD (2008) Investigation on the correlations between droplet and particle size distribution in ultrasonic spray pyrolysis. Ind Eng Chem Res 47:1650–1659. https://doi.org/10.1021/ie070821d
Hirano T, Kikkawa J, Shimokuri D, Nandiyanto ABD, Ogi T (2021) Sinter-necked, mixed nanoparticles of metallic tungsten and tungsten oxide produced in fuel-rich methane/air tubular flames. J Chem Eng Jpn 54:557–565. https://doi.org/10.1252/jcej.21we009
Hirano T, Nakakura S, Rinaldi FG, Tanabe E, Wang W-N, Ogi T (2018) Synthesis of highly crystalline hexagonal cesium tungsten bronze nanoparticles by flame-assisted spray pyrolysis. Adv Powder Technol 29:2512–2520. https://doi.org/10.1016/j.apt.2018.07.001
Zaouk D, Zaatar Y, Khoury A, Llinares C, Charles JP, Bechara J (2000) Fabrication of tin oxide (SnO2) thin film by electrostatic spray pyrolysis. Microelectron Eng 51–52:627–631. https://doi.org/10.1016/s0167-9317(99)00526-2
Chen C (1995) Fabrication of LiCoO2 thin film cathodes for rechargeable lithium battery by electrostatic spray pyrolysis. Solid State Ionics 80:1–4. https://doi.org/10.1016/0167-2738(95)00140-2
Mueller R, Mädler L, Pratsinis SE (2003) Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem Eng Sci 58:1969–1976. https://doi.org/10.1016/s0009-2509(03)00022-8
Wegner K, Schimmöller B, Thiebaut B, Fernandez C, Rao TN (2011) Pilot plants for industrial nanoparticle production by flame spray pyrolysis. KONA Powder Part J 29:251–265. https://doi.org/10.14356/kona.2011025
Cao KLA, Iskandar F, Tanabe E, Ogi T (2022) Recent advances in the fabrication and functionalization of nanostructured carbon spheres for energy storage applications. KONA Powder Part J:2023016. https://doi.org/10.14356/kona.2023016
Gradon L, Balgis R, Hirano T, Rahmatika AM, Ogi T, Okuyama K. (2020) Advanced aerosol technologies towards structure and morphologically controlled next-generation catalytic materials. J Aerosol Sci 149:105608. https://doi.org/10.1016/j.jaerosci.2020.105608
Jossen R, Pratsinis SE, Stark WJ, Madler L (2005) Criteria for flame-spray synthesis of hollow, shell-like, or inhomogeneous oxides. J Am Ceram Soc 88:1388–1393. https://doi.org/10.1111/j.1551-2916.2005.00249.x
Izumi F, Momma K (2007) Three-dimensional visualization in powder diffraction. Solid State Phenom 130:15–20. https://doi.org/10.4028/www.scientific.net/SSP.130.15
Halder NC, Wagner CNJ (1966) Separation of particle size and lattice strain in integral breadth measurements. Acta Crystallogr A 20:312–313. https://doi.org/10.1107/s0365110x66000628
Halder NC, Wagner CNJ (1966) Analysis of the broadening of powder pattern peaks using variance, integral breadth, and Fourier coefficients of the line profile. Adv X-Ray Anal 9:91–102
Balgis R, Widiyastuti W, Ogi T, Okuyama K (2017) Enhanced electrocatalytic activity of Pt/3D hierarchical bimodal macroporous carbon nanospheres. ACS Appl Mater Interfaces 9:23792–23799. https://doi.org/10.1021/acsami.7b05873
Tang J, Liu J, Torad NL, Kimura T, Yamauchi Y (2014) Tailored design of functional nanoporous carbon materials toward fuel cell applications. Nano Today 9:305–323. https://doi.org/10.1016/j.nantod.2014.05.003
Bott-Neto JL, Asset T, Maillard F, Dubau L, Ahmad Y, Guérin K, Berthon-Fabry S, Mosdale A, Mosdale R, Ticianelli EA, Chatenet M (2018) Utilization of graphitized and fluorinated carbon as platinum nanoparticles supports for application in proton exchange membrane fuel cell cathodes. J Power Sources 404:28–38. https://doi.org/10.1016/j.jpowsour.2018.10.004
Asset T, Chattot R, Maillard F, Dubau L, Ahmad Y, Batisse N, Dubois M, Guérin K, Labbé F, Metkemeijer R, Berthon-Fabry S, Chatenet M (2018) Activity and durability of platinum-based electrocatalysts supported on bare or fluorinated nanostructured carbon substrates. J Electrochem Soc 165:F3346–F3358. https://doi.org/10.1149/2.031806jes
Gokulakrishnan V, Parthiban S, Jeganathan K, Ramamurthi K (2011) Investigations on the structural, optical and electrical properties of Nb-doped SnO2 thin films. J Mater Sci 46:5553–5558. https://doi.org/10.1007/s10853-011-5504-x
Szczuko D, Werner J, Oswald S, Behr G, Wetzig K (2001) XPS investigations of surface segregation of doping elements in SnO2. Appl Surf Sci 179:301–306. https://doi.org/10.1016/s0169-4332(01)00298-7
Yamanaka T, Kurashima R, Mimaki J (2000) X-ray diffraction study of bond character of rutile-type SiO2, GeO2 and SnO2. Z Kristallogr - Cryst Mater 215:424–428. https://doi.org/10.1524/zkri.2000.215.7.424
Acknowledgements
This work was partly supported by the Center for Functional Nano Oxides at Hiroshima University, the International Network on Polyoxometalate Science, the JSPS Core-to-Core Program. The authors thank the Natural Science Center for Basic Research and Development (N-BARD) for help with SEM image measurements.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP22K20482 (T.H) and JP19H02500 (T.O.). This work was partly supported by the Information Center of Particle Technology, Japan, the Hosokawa Powder Technology Foundation, and the Kato Foundation for the Promotion of Science (KS-3229).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection: “Self-assembled Functional Nanomaterials and Devices in Asia”
Guest Editors: Zhixiang Wei and Yong Yan
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hirano, T., Tsuboi, T., Cao, K.L.A. et al. High specific surface area niobium-doped tin oxide nanoparticles produced in spray flames as catalyst supports in polymer electrolyte fuel cells. J Nanopart Res 25, 1 (2023). https://doi.org/10.1007/s11051-022-05649-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05649-3