Abstract
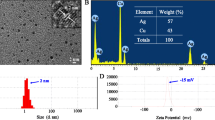
Silica Nanoparticles (SNPs) have been found to exhibit higher cytotoxicity to various cancer cells than to normal cells, while the underlying mechanisms are not fully understood. Here, SNPs triggered much higher cytotoxicity and apoptosis rate in human hepatoma HepG2 cells than in their normal counterparts L-02 cells; we thus selected these two cell lines as the cell model to investigate the mechanisms involved in the SNP-induced selective toxicity to cancer cells. Although uptake pathways and cellular trafficking of SNPs in HepG2 and L-02 cells were similar, more SNPs were taken up and accumulated in the mitochondria of cancer cells. After the removal of free SNPs from the culture medium, nanoparticles were excreted from HepG2 cells more effectively in the first 24 h, but 72 h later more SNPs still remained in cancer cells, leading to the continuous drop in cell viability of HepG2 cells. SNPs triggered a higher ROS generation, along with a lower intracellular GSH content and CAT activity in HepG2 cells than in L-02 cells. This could be due to the fact that HepG2 cells showed a much lower tolerance to H2O2-induced oxidative stress and cell death. Thus, the selective cytotoxicity of SNPs towards cancer cells could probably be explained by the higher particle uptake efficiency and cell sensitivity to oxidative stress as observed in HepG2 cells.







Similar content being viewed by others
Data availability
All data that support the findings of this study are included within the article.
References
Kirtane AR, Verma M, Karandikar P (2021) Nanotechnology approaches for global infectious diseases. Nat Nanotechnol 16(4):369–384
Salamanca-Buentello F, Daar AS (2021) Nanotechnology, equity and global health. Nat Nanotechnol 16(4):358–361
Jeelani PGH, Mulay P, Venkat R, Ramalingam C (2020) Multifaceted application of silica nanoparticles. A review Silicon 12(6):1337–1354
Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Zivcak M, Ghorbanpour M (2019) Application of silicon nanoparticles in agriculture. 3 Biotech 9(3):1–11
Yang Y, Zhang M, Song H, Yu C (2020) Silica-based nanoparticles for biomedical applications: from nanocarriers to biomodulators. Acc Chem Res 53(8):1545–1556
Tokgun O, Demiray A, Kaya B, Karagur E, Demir E, Burunkaya E, Akca H (2015) Silica nanoparticles can induce apoptosis via dead receptor and caspase 8 pathway on A549 cells. Adv Food Sci 37:65–70
Krętowski R, Kusaczuk M, Naumowicz M, Kotyńska J, Szynaka B, Cechowska-Pasko M (2017) The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials 7(8):230
Niu Y, Tang E, Zhang Q (2019) Cytotoxic effect of silica nanoparticles against hepatocellular carcinoma cells through necroptosis induction. Toxicol Res 8(6):1042–1049
Yu Y, Duan J, Yu Y, Li Y, Liu X, Zhou X, Ho K, Tian L, Sun Z et al (2014) Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J Hazard Mater 270:176–186
Lu X, Qian J, Zhou H, Gan Q, Tang W, Lu J, Yuan Y, Liu C (2011) In vitro cytotoxicity and induction of apoptosis by silica nanoparticles in human HepG2 hepatoma cells. Int J Nanomedicine 6:1889–1901
Beddoes CM, Case CP, Briscoe WH (2015) Understanding nanoparticle cellular entry: a physicochemical perspective. Adv Colloid Interface Sci 218:48–68
Malugin A, Herd H, Ghandehari H (2011) Differential toxicity of amorphous silica nanoparticles toward phagocytic and epithelial cells. J Nanopart Res 13(10):5381–5396
Blechinger J, Bauer AT, Torrano AA, Gorzelanny C, Brauchle C, Schneider SW et al (2013) Uptake kinetics and nanotoxicity of silica nanoparticles are cell type dependent. Small 9(23):3970–3980
Perez-Garnes M, Gutierrez-Salmeron M, Morales V, Chocarro-Calvo A, Sanz R, Garcia-Jimenez C, Garcia-Munoz RA et al (2020) Engineering hollow mesoporous silica nanoparticles to increase cytotoxicity. Mater Sci Eng C 112:110935
Chu ZQ, Huang YJ, Tao Q, Li Q (2011) Cellular uptake, evolution, and excretion of silica nanoparticles in human cells. Nanoscale 3(8):3291–3299
Al-Rawi M, Diabate S, Weiss C (2011) Uptake and intracellular localization of submicron and nano-sized SiO2 particles in HeLa cells. Arch Toxicol 85(7):813–826
He Q, Zhang Z, Gao Y, Shi J, Li Y (2009) Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small 5(23):2722–2729
Slowing II, Vivero-Escoto JL, Zhao Y, Kandel K, Peeraphatdit C, Trewyn BG (2011) Exocytosis of mesoporous silica nanoparticles from mammalian cells: from asymmetric cell-to-cell transfer to protein harvesting. Small 7(11):1526–1532
Yanes RE, Tarn D, Hwang AA, Ferris DP, Sherman SP, Thomas CR (2013) Involvement of lysosomal exocytosis in the excretion of mesoporous silica nanoparticles and enhancement of the drug delivery effect by exocytosis inhibition. Small 9(5):697–704
Wu Y, Tang W, Wang P, Liu C, Yuan Y, Qian J (2015) Cytotoxicity and cellular uptake of amorphous silica nanoparticles in human cancer cells. Part Part Syst Charact 32(7):779–787
Lehman SE, Morris AS, Mueller PS, Salem AK, Grassian VH, Larsen SC (2016) Silica nanoparticle-generated ROS as a predictor of cellular toxicity: mechanistic insights and safety by design. Environ Sci Nano 3(1):56–66
Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J (2009) Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol In Vitro 23(5):808–815
Ye Y, Liu J, Xu J, Sun L, Chen M, Lan M (2010) Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol In Vitro 24(3):751–758
Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12(1):376–390
Moloney JN, Cotter TG (2018) ROS signalling in the biology of cancer. Semin Cell Dev Biol 80:50–64
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med 52(2):192–203
Reczek CR, Chandel NS (2017) The two faces of reactive oxygen species in cancer. Annu Rev Biochem 1:79–98
Cho HD, Lee JH, Moon KD, Park KH, Lee MK, Seo KI (2018) Auriculasin-induced ROS causes prostate cancer cell death via induction of apoptosis. Food Chem Toxicol 111:660–669
Raza MH, Siraj S, Arshad A, Waheed U, Aldakheel F, Alduraywish S (2017) ROS-modulated therapeutic approaches in cancer treatment. J Cancer Res Clin 143(9):1789–1809
Inoue M, Sakamoto K, Suzuki A, Nakai S, Ando A, Shiraki Y (2021) Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part Fibre Toxicol 18(1):1–20
Lin W, Huang YW, Zhou XD, Ma Y (2006) In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol 217(3):252–259
Zhu J, Liao L, Zhu L, Zhang P, Guo K, Kong J (2013) Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta 107:408–415
Shahabi S, Treccani L, Dringen R, Rezwan K (2015) Dual fluorophore doped silica nanoparticles for cellular localization studies in multiple stained cells. Acta Biomater 14:208–216
Veeranarayanan S, Poulose AC, Mohamed S, Aravind A, Nagaoka Y, Yoshida Y (2012) FITC labeled silica nanoparticles as efficient cell tags: uptake and photostability study in endothelial cells. J Fluoresc 22(2):537–548
Li J, Tian J, Yin H, Peng Y, Liu S, Yao S, Zhang L (2021) Chemical conjugation of FITC to track silica nanoparticles in vivo and in vitro: an emerging method to assess the reproductive toxicity of industrial nanomaterials. Environ Int 152:106497
Saikia J, Yazdimamaghani M, Hadipour Moghaddam SP, Ghandehari H (2016) Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS Appl Mater Interfaces 8(50):34820–34832
Li L, Xi WS, Su Q, Li Y, Yan GH, Liu Y (2019) Unexpected size effect: the interplay between different-sized nanoparticles in their cellular uptake. Small 15(38):1901687
Glorani G, Marin R, Canton P, Pinto M, Conti G, Fracasso G (2017) Pegylated silica nanoparticles: cytotoxicity and macrophage uptake. J Nanopart Res 19(8):1–14
Hu L, Mao Z, Zhang Y, Gao C (2011) Influences of size of silica particles on the cellular endocytosis, exocytosis and cell activity of HepG2 cells. J Nanosci Lett 1:1–16
Ivosev V, Sanchez GJ, Stefancikova L, Haidar DA, Gonzalez Vargas CR, Yang X (2020) Uptake and excretion dynamics of gold nanoparticles in cancer cells and fibroblasts. Nanotechnology 31(13):135102
Qi Y, Ma R, Li X, Lv S, Liu X, Abulikemu A (2020) Disturbed mitochondrial quality control involved in hepatocytotoxicity induced by silica nanoparticles. Nanoscale 12(24):13034–13045
Menon N, Leong DT (2016) Cytotoxic effects of phosphonate-functionalized mesoporous silica nanoparticles. Acs Appl Mater Inter 8(3):2416–2422
Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X (2011) Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett 11(2):772–780
Guo CX, Yang M, Jing L, Wang J, Yu Y, Li Y (2016) Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int J Nanomedicine 11(2016):5257–5276
Kusaczuk M, Kretowski R, Naumowicz M, Stypulkowska A, Cechowska-Pasko M (2018) Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int J Nanomedicine 13:2279–2294
Ahmad J, Ahamed M, Akhtar MJ, Alrokayan SA, Siddiqui MA, Musarrat J (2012) Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharmacol 259(2):160–168
Sabharwal SS, Schumacker PT (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 14(11):709–721
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–947
Wang J, Yi J (2008) Cancer cell killing via ROS To increase or decrease, that is the question. Cancer Biol Ther 7(12):1875–1884
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10(3):241–252
Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33(5):1477–1488
Kong N, Ji X, Wang J, Sun X, Chen G, Fan T (2020) ROS-mediated selective killing effect of black phosphorus: mechanistic understanding and its guidance for safe biomedical applications. Nano Lett 20(5):3943–3955
Berg JM, Romoser AA, Figueroa DE, Spencer West C, Sayes CM (2013) Comparative cytological responses of lung epithelial and pleural mesothelial cells following in vitro exposure to nanoscale SiO2. Toxicol In Vitro 27(1):24–33
Kung ML, Hsieh SL, Wu CC, Chu TH, Lin YC, Yeh BW (2015) Enhanced reactive oxygen species overexpression by CuO nanoparticles in poorly differentiated hepatocellular carcinoma cells. Nanoscale 7(5):1820–1829
Kim IY, Joachim E, Choi H, Kim K (2015) Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine 11(6):1407–1416
Funding
This study is supported by the National Key Research and Development Program of China (2020YFA0908900), the National Natural Science Foundation of China (31871011).
Author information
Authors and Affiliations
Contributions
Peng Wang: Investigation, Original draft.
Tao Shen: Formal analysis, Writing—original draft.
Yi Sun: Data Curation.
Xinhui Cui: Validation.
Changsheng Liu: Resources, Supervision, Funding acquisition.
Yuan Yuan: Methodology, Supervision.
Jiangchao Qian: Conceptualization, Methodology, Writing—review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Shen, T., Sun, Y. et al. Selective killing of cancer cells by silica nanoparticles due to increased nanoparticle internalization and cellular sensitivity to oxidative stress. J Nanopart Res 25, 13 (2023). https://doi.org/10.1007/s11051-022-05643-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05643-9