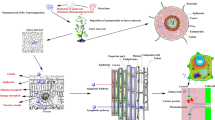
Abstract
The production and use of nanoparticles (NPs) have increased dramatically in recent decades, releasing them into the environment. Pollution of soil, water, and air with these substances affects growth and development of all types of living organisms specially the plants because they are immobile and cannot move away from environmental stresses. Among the most commonly used NPs with a great ecological impact are ZnO NPs. In the present work, we studied the effects of different concentrations of ZnO NPs (0, 25, 50, 100, and 200 mg/L) on an important crop plant, Nicotiana tabacum L., which is also a commonly used model organism in the research of abiotic stress, using biochemical, physiological, and molecular approaches and compared them with the effects of bulk ZnO particles. According to the results, the form of particles and their concentration play an important role in response of plants to the treatment conditions. In fact, ZnO NPs had relatively more pronounced impacts than bulk particles on most plant features, and this was dose dependently. It was observed that ZnO NPs in lower concentration (25 mg/L) provoked the plant growth more than bulk particles which was in accordance with higher photosynthetic pigments content observed in this concentration. Furthermore, the expression of photosynthesis-related genes, i.e., CHLΙ, LHCa/b, and RSSU, as well as the amount of IAA and GA phytohormones, was the highest at 25 mg/L NPs. Further studies are required to better understand the exact mechanism of action of these particles including their entrance mode in to the plant cell and cellular localization, their transportation within the whole plant, and the molecular pathways affected by these particles.








Similar content being viewed by others
References
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Faizan M, Hayat S, Pichtel J (2020) Effects of zinc oxide nanoparticles on crop plants: a perspective analysis. Agriculture Review 41:83–99. https://doi.org/10.1007/978-3-0.30-33996-8-4
Cvjetko P, Zovko M, Stefanic PP, Biba R, Tkalec M, Domijan A-M, Vrcek IV, Letofsky-Papst I, Sikic S, Balen B (2017) Phytotoxic effects particles in tobacco plants. Environ Sci Pollut Res 25(6):5590–5602. https://doi.org/10.1007/s113561709288
Plaksenkova I, Kokina I, Petrova A, Jermalonoka M, Gerbreders V, Krasovska M (2020) The impact of zinc oxide nanoparticles on cytotoxicity, genotoxicity and miRNA expression in barley (Hordeum vulgare L.) seedlings. Sci World J. https://doi.org/10.1155/2020/6649746
Pandey AC, Sanjay SS, Yadav RS (2010) Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J Exp Nanosci 5:6488–6497. https://doi.org/10.1080/17458081003649648
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479. https://doi.org/10.1021/es901695c
Akansha S, Deepak G, Rakesh KS, Vishnu D, Tatiana M, Marina V, Sudhakar S, Manjoi S (2021) Effect on ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10:2556. https://doi.org/10.3390/plants10122556
Xia T, Li N, Nel AE (2009) Potential health impact of nanoparticles. Annu Rev Public Health 30:137–150. https://doi.org/10.1146/annurev.publhealth.031308.100155
Shvedova A, Kagan VE, Fadeel B (2010) Close encounters of the small kind: adverse effects of man-made materials interfacing with the nano-cosmos of biological systems. Ann Rev Pharmacol 50:63–88. https://doi.org/10.1146/annurev.pharmtox.010909.105819
Jitao LV, Peterchristie, (2019) Uptake, translocation and transformation of metal-based nanoparticles in plants: recent advanced and methodological challenges. Environ Sci Nano 6:4159. https://doi.org/10.1039/C8EN00645H
Amit K, Indrakant KS, Archana S (2021) The role of zinc oxide nanoparticles in plants: a critical appraisal.Nanomater Biointeraction Cell, Organismal Syst Level249–267. https://doi.org/10.1007/978-3-030-65792-5_10
Thounaojam TC, Meetei TT, Devi YB et al (2021) Zinc oxide nanoparticles (ZnO-NPs): a promising nanoparticle in renovating plant science. Acta Physiol Plant 43:136. https://doi.org/10.1007/s11738-021-03307-0
Peralta-Videal JR, Zhao L, Lopez-Moreno ML (2011) Nanomaterials and the environment: a review for the biennium. Natl Libr Med 186(1):1–15. https://doi.org/10.1016/j.jhazmat.2010.11.020
Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47:10637–11064. https://doi.org/10.1021/es402209w
Dietz K-J, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16(11):582–589. https://doi.org/10.1016/j.tplants.2011.08.003
Zhao LJ, Peralta-Videa J, Ren MH, Varela-Ramirez A, Hernandez-Viezcas JA, Aguilera RJ, Gardea Torresdey JL (2012) Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: electron microsprobe and confocal microscopy studies. Chem Eng J 184:1–8. https://doi.org/10.1016/j.cej.2012.01.041
Wang XP, QQ L, Pei ZM, Wang SC (2018) Effects of zinc oxide nanoparticles on the growth, photosynthetic traits and antioxidative enzymes in tomato plants. Biologia Plantarum 62:301808. https://doi.org/10.1007/s10535-018-0813-4
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408(16):3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031
Zhao L, Sun Y, Hernandez-Viezcas J, Servin AD, Hong J, Niu G (2013) Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J Agric Food Chem 61(49):11945–11951. https://doi.org/10.1021/jf404328e
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 50:243–250. https://doi.org/10.1016/j.envpol.2007.01.016
Wang P, Menzies NW, Lombi E, Mc Kenna BA, Johannessen B, Glover CJ (2013) Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ Sci Technol 42(23):13822–13830. https://doi.org/10.1021/es403466p
Ruangthep P, Samart S, Chutipaijit S (2018) ZnO nanoparticles affect differently the morphological and physiological responses of Riceberry plants (Oryza sativa L.). SNRU J Sci Technol 10(1):75–81
Lee CW, Mahendra S, Zodrow K, Tsai D, Braam PJJ (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem 29:669–675. https://doi.org/10.1002/etc.58
Lee S, Kim S, Lee I (2013) Assessment of phytotoxicity of ZnONPs on a medicinal plant, Fagopyrum esculentum. Environ Sci Pollut Res 20(2):848–854. https://doi.org/10.1007/s11356-012-1069-8
Thwala Musee N, Sikhwivhilu L, Wepener V (2013) The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctata and the role of testing media parameters. Environ Sci Proc Impacts 15:1830–1843. https://doi.org/10.1039/c3em00235g
Kouhi SMM, Lahouti M, Ganjeali A, Entezar MH (2014) Comparative microparticles, and Zn2+on rapeseed (Brassica napus L.): investigating a wide range of concentrations. Toxicol Environ Chem 96:861–868. https://doi.org/10.1080/02772248.2014.994517
Bandyopadhyay S, Plascencia-Villa G, Mukherjee A, Rico CM, Jose-Yacaman M, Peralta-Videa JR, Gardea- Torresdey JL (2015) Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ 15:515–516. https://doi.org/10.1016/j.scitotenv.2015.02.014
Wang XP, Jiang P, Ma Y, Geng SJ, Wang SC, Shi DC (2015) Physiological strategies of sunflower exposed to salt or alkali stresses: restriction of ion transport in the cotyledon node zone and solute accumulation. Agron J 107:2181–2192. https://doi.org/10.2134/agronj15.0012
Bagawade JA, Jagtap SS (2018) Effect of zinc oxide nanoparticles on germination and growth characteristics in wheat plants (Triticum aestivum L.). Int J Adv Eng Res Dev 13(1):315–323
Yoshihara S, Yamamoto K, Nakajima Y, Takeda S, Kurahashi K, Tokumoto H (2019) Absorption of zinc ions dissolved from zinc oxide nanoparticles in the tobacco callus improves plant productivity. Plant Cell, Tissue Organ Cult 138(2):1–9. https://doi.org/10.1007/s11240-019-01636-0
Hira Zafar, Joham Sarfaraz Ali, Attarad Ali (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Frontier in Plant Science 7(376). https://doi.org/10.3389/fpls.2016.00535
Iziy E, Majd A, Vaezi-Kakhki MR, Nejadsattari T, Noureini SK (2019) Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc Bot Pol 88(4):36–39. https://doi.org/10.5586/asbp.3639
Elshoky HA, Yotsova E, Farghali MA, Farroh KY (2021) Impact of foliar spray of zinc oxide nanoparticles on the photosynthesis of Pisum sativum L. under salt stress. Plant Physiol Biochem 167(10):607–618. https://doi.org/10.1016/j.plaphy.2021.08.039
Venkatachalam P, Mani J, Manikandan R, Geetha N (2016) Zinc oxide nanoparticles (ZnO NPs) alleviated heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69. https://doi.org/10.1016/j.plaphy.2016.08.022
Samart S, Phakamas N, Chutipaijit S (2014) Evaluating the effect of zinc oxide nanoparticles on the physiological responses of Thai rice (Oryza sativa L.). The 3rd Joint Conference on Renewable Energy and Nanotechnology. Mahidol University. Kanchanaburi Campus. Thailand. 2223 December 14
Han QH, Huang B, Ding CB (2017) Effect of melatonin and antioxidative system and photosystem П in cold-stressed Rice seedling. Front Plant Sci 8:785. https://doi.org/10.3389/fpls.2017.00785
Adhikari T, Kundu S, Rao AS (2016) Zinc delivery to plants through seed coating with nano-zinc oxide particles. J Plant Nutriton 39:136–146. https://doi.org/10.1080/01904167.2015.1087562
García-Lopez JI, Zavala-García F, Olivares-Saenz E, Lira-Saldívar R, Barriga-Castro ED, Ruiz-Torres NA, Ramos-Cortez E, Vazquez-Alvarado R, Nino-Medina G (2018) Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 82(10):215. https://doi.org/10.3390/agronomy8100215
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 1(115):677–702. https://doi.org/10.1111/j.1469-8137.2007.01996.x
Singh MV (2009) Micronutrient nutritional problems in soils of India and improvement for human and animal’s health. Indian J Fertil 5(4):11–26
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification. Plant Soil 302(1):1–17. https://doi.org/10.1007/s11104-007-9466-3
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. National library of medicine 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Pandey N, Pathak GC, Sharma CP (2006) Zinc is critically required for pollen function and fertilization in lentil. J Trace Elem Med Biol 20(2):89–96. https://doi.org/10.1016/j.jtemb.2005.09.006
Sun L, Song F, Zhu X, Liu S, Liu F, Wang Y, Li X (2020) Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Archiv Agron Soil Sci 67:245–259. https://doi.org/10.1080/03650340.2020.1723003
Lichtenthaler H, Wellburn A (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11(5):591–592. https://doi.org/10.1042/bst0110591
Sarropoulou V, Dimassi-Theriou K, Therios I, Koukourikou-Petridou M (2012) Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry root stock PHLC (Prunus avium-Prunus cerasus). Plant Physiol Biochem 61:162–168. https://doi.org/10.1016/j.plaphy.2012.10.001
Sachin B (2017) Inducible expression of magnesium protoporphyrin chelatase subunit I (CHLI)-amiRNA provides insights into cucumber mosaic virus Y satellite RNA-induced chlorosis symptoms. Virus eases 28(1):69–80. https://doi.org/10.1007/s13337-017-0360-1
Yuan XK, Yang ZQ (2018) The effect of endogenous hormones on plant morphology and fruit quality of tomato under difference between day and night temperature. Hort Sci (Prague) 45(3):131–138. https://doi.org/10.17221/7/2017-HORTSCI
Nair PMG, Chung IM (2017) Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings exposure. Sci Total Environ 575:187–198. https://doi.org/10.1016/j.scitotenv.2016.10.017
Tognetti VB, Mühlenbock PER, Van Breusegem F (2012) Stress homeostasis-the redox and auxin perspective. Plant, Cell Environ 35:321–333. https://doi.org/10.1111/j.13653040.2011.02324.x
Vishal B, Kumar PP (2018) Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Frontirs in Plant Science 9:838. https://doi.org/10.3389/fpls.2018.00838
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J Biol Sci 21(1):13–17. https://doi.org/10.1016/j.sjbs.2013.04.005
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, Gardea Torresde JL (2014) Physiological effect of nanoparticles ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138. https://doi.org/10.1039/c3mt00064h
Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30(10):499–511. https://doi.org/10.1016/j.tibtech.2012.06.004
Darlington TK, Neigh AM, Spencer MT (2009) Nanoparticle characteristic affecting environmental fate and transport through soil. Environ Toxicol 28:1191–1199. https://doi.org/10.1897/08-341.1
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828. https://doi.org/10.1039/c0em00611d
El-Kereti M, El-feky S, Khater M, Osman Y, El-sherbini E (2013) ZnO nanofertilizer and He Ne laser irradiation for promoting growth and yield of sweet basil plant. Recent Patent Food Nutr Agric 5:169–181. https://doi.org/10.2174/2212798405666131112142517
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2(1):4857. https://doi.org/10.1007/s40003-012-0049-z
Subbaiah LV, Prasad TNVKV, Krishna TG, Sudhakar P, Reddy BR, Pradeep T (2016) Novel effects of nanoparticulate delivery of zinc on growth, productivity and zinc biofortification in maize (Zea mays L.). J Agr Food Chem 64:3778–3788. https://doi.org/10.1021/acs.jafc.6b00838
Mazaheri Tirani M, Madadkar M (2019) Hydroponic grown tobacco plants respond to zinc oxide nanoparticle and bulk exposure morphological, physiological and anatomical adjustment. Funct Plant Biol 46(4):360–375. https://doi.org/10.1071/FP18076
Mousavi Kouhi SM, Lahouti M, Gangeali A, Entezari M (2015) Long term exposure of rapeseed (Brassica napus L.) to ZnO nanoparticles: anatomical and ultrastructural response. Environ Sci Pollut Res 11. https://doi.org/10.1007/s11356-015-4306-0
Akhavan Hezaveh T, Pourakbar L, Rahmani F, Alipour H (2020) Effect of ZnO NPs on phenolic compound of rapeseed under salinity stress. J Plant Process Funct 8(34):11–18
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 9618–9198. https://doi.org/10.1016/j.plaphy.2015.07.026
Li X, Yang Y, Jia L, Chen H, Wei X (2013) Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf 89:150–157. https://doi.org/10.1016/j.ecoenv.2012.11.025
Martinez JP, Silva H, Ledent F, Pinto M (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26(1):30–38. https://doi.org/10.1016/j.eja.2006.08.003
S Narendhran, P Rajiv, Rajeshwari Sivaraj (2016) Influence of zinc oxide nanoparticles on growth of Sesamum indicum L. in zinc deficient soil. Int J Pharm Pharm Sci 8:365–381. ISSN:0975–1491
Singh NB, Amist N, Yadav K, Singh D, Pandey JK, Singh SC (2013) Zinc oxide nanoparticles as fertilizer for the germination, growth and metabolism of vegetable crops. J Nanoeng Nanomanuf 3:353364. https://doi.org/10.1166/jnan.2013.1156
García-Sanchez S, Bernales I, Cristobal S (2015) Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics 16:341. https://doi.org/10.1186/s12864-015-1530-4
Connell SL, Al-Hamdani SH (2001) Selected physiological responses of kudzu to different chromium concentrations. Can J Plant Sci 81:53–58. https://doi.org/10.4141/P00-029
Rao S, Shekhawat GS (2014) Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea. J Environ Chem Eng 2:105–114. https://doi.org/10.1016/j.jece.2013.11.029
Demidchik V (2015) Mechanism of oxidative stress in plants: from chemical chemistry to cell biology. Environ Exp Bot 109:212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Devlin MR, Withman FH (2002) Plant physiology. CBs publishers and distributers. Chapter 12. Fire lettuce. Physiol Planetarium 103:1–7
Xp Wang X, Yang SC, Li Q, Wang W, Hou C, Gao X, Wang Li, Wang S (2016) Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plant Sci 6:1243. https://doi.org/10.3389/fpls.2015.01243
Prasad T, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927. https://doi.org/10.1080/01904167.2012.663443
Wang F, Liu X, Shi Z, Tong R, Adams CA, Shi X (2015) Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants-a soil microcosm experiment. Chemosphere 147:88–97. https://doi.org/10.1016/j.chemosphere.2015.12.076
Boykov IN, Shuford E, Zhang H (2019) Nanoparticle titanium dioxide affects the growth and microRNA expression of switchgrass (Panicum virgatum). Genomics 111:450–456. https://doi.org/10.1016/j.ygeno.2018.03.002
Petrova A, Plaksenkova I, Kokina I, JermalOnoka M (2021) Effect of Fe3O4 and CuO nanoparticles on morphology, genotoxicity, and miRNA expression on different barley (Hordeum vulgare L.) genotypes. Sci World J 2021:1–11. https://doi.org/10.1155/2021/6644689
Zhang B (2015) MicroRNA: a new target for improving plant tolerance to abiotic stress. J Exp Bot 66(7):1749–1761. https://doi.org/10.1093/jxb/erv013
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307. https://doi.org/10.1038/nchembio.165
Ahammed G, Xia X, Li X, Shi K, Yu J, Zhou Y (2014) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16:462–473. https://doi.org/10.2174/1389203716666150330141427
Hafeeze B, Khanife YM, Saleem M (2013) Role of zinc in plant nutrition-a review. Am J Exp Agric 3(2):374–391
Upreti KK, Sharma M (2016) Role of plant growth regulators in abiotic stress tolerance. Abiotic Stress Physiol Hort Crops 150:414–418. https://doi.org/10.1007/978-81-322-2725-0-2
Shu K, Zhou W, Chen F, Luo X, Yang W (2018) Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Fron Plant Sci 9:1–8. https://doi.org/10.3389/fpls.2018.00416
Vankova R, Landa P, Podlipna R, Petre I, Prerostova S, Langhansova L, Gaudinova A, Motkova K, Knirsch V, Vanek T (2017) ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Science of the Total Environment 535-542. https://doi.org/10.1016/j.scitotenv.2017.03.160
Farrag HF (2015) Evaluation of the growth responses of Lemna gibba L. (Duckweed) to silver and zinc oxide nanoparticles. World Appl Sci J 33(2):190- exposed 202. https://doi.org/10.5829/idosi.wasj.2015.33.02.83
Amin MA, Badawy AA (2017) Metabolic changes in common bean plants in response to zinc nanoparticles and zinc sulfate. IJISET-Int J Innov Sci, Eng Technol 4(5)
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/j.plaphy.2014.07.010
Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5:379–391. https://doi.org/10.1038/nrm1364
Dayan J, Voronin N, Gong F, Sun T-p, Hedden P, Fromm H, Aloni R (2012) Leaf-induced gibberellin signaling is essential for internode elongation, cambial activity, and fiber differentiation in tobacco stems. Plant Cell 24(1):66–79. https://doi.org/10.1105/tpc.111.093096
Kleckerova A, Sobrova P, Krystofova O, Sochor J, Zitka O, Babula P, Adam V, Docekalova H, Kizek R (2011) Cadmium (II) and zinc (II) ions effects on maize plants revealed by spectroscopy and electrochemistry. Int J Electrochem Sci 6:6011–6031
Shi WG, Li H, Liu TX, Polle A, Peng CH, Luo ZB (2015) Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus x canescens exposed to excess zinc. Plant Cell Environ 38:207–223. https://doi.org/10.1111/pce.12434
Chaca P, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.). Merr Acta Physiol Plant 36:2815–2826. https://doi.org/10.1007/s11738-014-1656-z
Yang J, Cao W, Rui Y (2017) Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact 12(1):158–169. https://doi.org/10.1080/17429145.2017.1310944
Chen BJW, Hajiboland R, Bahrami-Rad S, Moradtalab N, Anten NPR (2019) Presence of below ground neighbors activates defense pathways at the expense of growth in tobacco plants. Front Plant Sci 10:751. https://doi.org/10.3389/fpls.2019.00751
Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A (2010) ABA promotes quiescence of the quiescent center and suppressed stem cell differentiation in the Arabidopsis primary root meristem. Plant J 64:764–774. https://doi.org/10.1111/j.1365-313x.2010.04367.x
Funding
The authors would like to thank the University of Tabriz for financial support of this project under Grant no. 52/418454/1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mardi, A., Mohajjel Shoja, H. & Mohajel Kazemi, E. Comparative study of growth responses, photosynthetic pigment content, and gene expression pattern in tobacco plants treated with ZnO nano and ZnO bulk particles. J Nanopart Res 24, 208 (2022). https://doi.org/10.1007/s11051-022-05583-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05583-4