Abstract
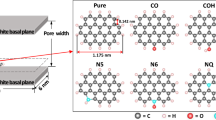
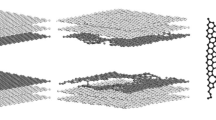
In this paper, the grand canonical Monte Carlo (GCMC) method was used to explore the effects of four pore structures (disordered pore, wedge pore, carbon nanotube, and slit pore structures) and surface curvature of activated carbon on the adsorption and separation of CO2/N2. On the whole, carbon nanotubes have the greatest selectivity for CO2, followed by disordered pores, wedge pores, and slit pores. The effect of pore structure on the interaction energy of gas molecules is similar to that of selectivity, in which the fluid–solid potential energy between adsorbates and adsorbents plays an important role. Due to the different affinity between adsorbate molecules and activated carbon, CO2 with high affinity is more sensitive to the change of pore size. Therefore, under high pressure, the density of CO2 in the slit pore is greater than that in the wedge pore. However, N2 with poor affinity is limited by the surface area, resulting in the density of it in the wedge pore is always higher than that in the slit pore. Although the existence of non-six membered corannulene rings in activated carbon can’t always cause the increase of specific surface area, the surface curvature of activated carbon caused by it can increase strong energetically adsorption sites. Hence, the surface curvature plays a positive role in the adsorption density, interaction energy, and CO2 selectivity. The discovery of CO2/N2 adsorption and separation at the molecular level is expected to provide valuable insights.

















Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mukherjee S, Sikdar N, O’Nolan D, Franzvictoria DM, Gascon V, Kumar A, Kumar N, Scott HS, Madden DG, Kruger PE, Space B, Zaworotko MJ (2019) Trace CO2 capture by an ultramicroporous physisorbent with low water affinity. Sci Adv 5: eaax9171. https://doi.org/10.1126/sciadv.aax9171
Kim EJ, Siegelman RL, Jiang HZH, Forse AC, Lee JH, Martell JD, Milner PJ, Falkowski JM, Neaton JB, Reimer JA, Weston SC, Long JR (2020) Cooperative carbon capture and steam regeneration with tetraamine-appended metal–organic frameworks. Science 369:392–396. https://doi.org/10.1126/science.abb3976
Gao W, Liang S, Wang R, Jiang Q, Zhang Y, Zheng Q, Xie B, Toe CY, Zhu X, Wang J, Huang L, Gao Y, Wang Z, Jo C, Wang Q, Wang L, Liu Y, Louis B, Scott J, Roger AC, Amal R, He H, Park SE (2020) Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chem Soc Rev 49:8584–8686. https://doi.org/10.1039/D0CS00025F
Li X, Yan B, Huang W, Bian H, Wang X, Zhu J, Dong S, Wang Y, Chen W (2022) Room-temperature synthesis of hydrophobic/oleophilic ZIF-90-CF3/melamine foam composite for the efficient removal of organic compounds from wastewater. Chem Eng J 428:132501. https://doi.org/10.1016/j.cej.2021.132501
Jia W, Li Q, Zhang L, Hou L, Liu T, Bhavana G, Yang J (2020) Highly efficient photocatalytic reduction of CO2 on amine-functionalized Ti-MCM-41 zeolite. J Nanopart Res 22:1–9. https://doi.org/10.1007/s11051-020-05019-x
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511. https://doi.org/10.1016/j.cej.2012.12.038
Chu Y, Khan MA, Zhu S, Xia M, Lei W, Wang F, Xu Y (2019) Microstructural modification of organo-montmorillonite with Gemini surfactant containing four ammonium cations: molecular dynamics (MD) simulations and adsorption capacity for copper ions. J Chem Technol Biot 94:3585–3594. https://doi.org/10.1002/jctb.6162
Fu L, Zhu J, Huang W, Fang J, Sun X, Wang X, Liao K (2020) Preparation of nano-porous carbon-silica composites and its adsorption capacity to volatile organic compounds. Processes 8:372. https://doi.org/10.3390/pr8030372
Tran C, Kalra V (2013) Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J Power Sources 235:289–296. https://doi.org/10.1016/j.jpowsour.2013.01.080
Shen C, Grande CA, Li P, Yu J, Rodrigues AE (2010) Adsorption equilibria and kinetics of CO2 and N2 on activated carbon beads. Chem Eng J 160:398–407. https://doi.org/10.1016/j.cej.2009.12.005
Yi H, Li F, Ning P, Tang X, Peng J, Li Y, Deng H (2013) Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem Eng J 215:635–642. https://doi.org/10.1016/j.cej.2012.11.050
Liu L, Nicholson D, Bhatia SK (2015) Adsorption of CH4 and CH4/CO2 mixtures in carbon nanotubes and disordered carbons: a molecular simulation study. Chem Eng Sci 121:268–278. https://doi.org/10.1016/j.ces.2014.07.041
Kumar KV, Rodríguez-Reinoso F (2012) Effect of pore structure on the selectivity of carbon materials for the separation of CO2/H2 mixtures: new insights from molecular simulation. RSC Adv 2:9671–9678. https://doi.org/10.1039/C2RA20775C
Fan C, Do DD, Nicholson D (2013) Condensation and evaporation in capillaries with nonuniform cross sections. Ind Eng Chem Res 52:14304–14314. https://doi.org/10.1021/ie402549z
Li L, Liu S, Liu J (2011) Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J Hazard Mater 192:683–690. https://doi.org/10.1016/j.jhazmat.2011.05.069
Zeng Y, Phadungbut P, Do DD, Nicholson D (2015) Wedge pore model as an alternative to the uniform slit pore model for the determination of pore size distribution in activated carbon. J Phys Chem C 119:25853–25859. https://doi.org/10.1021/acs.jpcc.5b06085
Bhatia SK (2017) Characterizing structural complexity in disordered carbons: from the slit pore to atomistic models. Langmuir 33:831–847. https://doi.org/10.1021/acs.langmuir.6b03459
Agnihotri S, Mota JPB, Rostam-Abadi M, Rood MJ (2005) Structural characterization of single-walled carbon nanotube bundles by experiment and molecular simulation. Langmuir 21:896–904. https://doi.org/10.1021/la047662c
Kumar KV, Müller EA, Rodriguez-Reinoso F (2012) Effect of pore morphology on the adsorption of methane/hydrogen mixtures on carbon micropores. J Phys Chem C 116:11820–11829. https://doi.org/10.1021/jp302749t
Zhang Z, Brydson R, Aslam Z, Reddy S, Brown A, Westwood A, Rand B (2011) Investigating the structure of non-graphitising carbons using electron energy loss spectroscopy in the transmission electron microscope. Carbon 49:5049–5063. https://doi.org/10.1016/j.carbon.2011.07.023
Acharya M, Strano MS, Mathews JP, Billinge SJL, Petkov V, Subramoney S, Foley HC (1999) Simulation of nanoporous carbons: a chemically constrained structure. Philos Mag B 79:1499–1518. https://doi.org/10.1080/13642819908218318
Wiśniewski M, Werengowska-Ciećwierz K, Terzyk AP (2015) New findings on the influence of carbon surface curvature on energetics of benzene adsorption from aqueous solutions. Chem Phys Lett 619:219–222. https://doi.org/10.1016/j.cplett.2014.11.024
Klauda JB, Jiang J, Sandler SI (2004) An ab initio study on the effect of carbon surface curvature and ring structure on N2 (O2)− carbon intermolecular potentials. J Phys Chem B 108:9842–9851. https://doi.org/10.1021/jp037897h
Balamurugan K, Singam ERA, Subramanian V (2011) Effect of curvature on the α-helix breaking tendency of carbon based nanomaterials. J Phys Chem C 115:8886–8892. https://doi.org/10.1021/jp110898r
Harris PJF (2013) Fullerene-like models for microporous carbon. J Mater Sci 48:565–577. https://doi.org/10.1007/s10853-012-6788-1
Bahamon D, Ogungbenro AE, Khaleel M, Abu-Zahra MRM, Vega LF (2020) Performance of activated carbons derived from date seeds in CO2 swing adsorption determined by combining experimental and molecular simulation data. Ind Eng Chem Res 59:7161–7173. https://doi.org/10.1021/acs.iecr.9b05542
Li S, Song K, Zhao D, Rugarabamu JR, Diao R, Gu Y (2020) Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Micropor Mesopor Mat 302:110220. https://doi.org/10.1016/j.micromeso.2020.110220
Lucena SMP, Paiva CAS, Silvino PFG, Azevedo DCS Jr, CLC, (2010) The effect of heterogeneity in the randomly etched graphite model for carbon pore size characterization. Carbon 48:2554–2565. https://doi.org/10.1016/j.carbon.2010.03.034
Leyssale JM, Da CJP, Germain C, Weisbecker P, Vignoles GL (2012) Structural features of pyrocarbon atomistic models constructed from transmission electron microscopy images. Carbon 50:4388–4400. https://doi.org/10.1016/j.carbon.2012.05.015
Park Y, Moon DK, Kim YH, Ahn H, Lee CH (2014) Adsorption isotherms of CO2, CO, N2, CH4, Ar and H2 on activated carbon and zeolite LiX up to 1.0 MPa. Adsorption 20:631–647. https://doi.org/10.1007/s10450-014-9608-x
An Y, Fu Q, Zhang D, Wang Y, Tang Z (2019) Performance evaluation of activated carbon with different pore sizes and functional groups for VOC adsorption by molecular simulation. Chemosphere 227:9–16. https://doi.org/10.1016/j.chemosphere.2019.04.011
Liao PQ, Zhang WX, Zhang JP, Chen XM (2015) Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat Commun 6:1–9. https://doi.org/10.1038/ncomms9697
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865. https://doi.org/10.1103/PhysRevLett.77.3865
Harris JG, Yung KH (1995) Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J Phys Chem 99:12021–12024. https://doi.org/10.1021/j100031a034
Makrodimitris K, Papadopoulos GK, Theodorou DN (2001) Prediction of permeation properties of CO2 and N2 through silicalite via molecular simulations. J Phys Chem B 105:777–788. https://doi.org/10.1021/jp002866x
Mayo SL, Olafson BD, Goddard WA (1990) DREIDING: a generic force field for molecular simulations. J Phys Chem 94:8897–8909. https://doi.org/10.1021/j100389a010
Kumar KV, Preuss K, Lu L, Guo ZX, Titirici MM (2015) Effect of nitrogen doping on the CO2 adsorption behavior in nanoporous carbon structures: a molecular simulation study. J Phys Chem C 119:22310–22321. https://doi.org/10.1021/acs.jpcc.5b06017
Mukherjee A, Okolie JA, Abdelrasoul A, Niu C, Dalai AK (2019) Review of post-combustion carbon dioxide capture technologies using activated carbon. J Environ Sci 83:46–63. https://doi.org/10.3390/pr8030372
Hong SM, Choi SW, Kim SH, Lee KB (2016) Porous carbon based on polyvinylidene fluoride: Enhancement of CO2 adsorption by physical activation. Carbon 99:354–360. https://doi.org/10.1016/j.carbon.2015.12.012
Zhuo S, Huang Y, Hu J, Liu H, Hu Y, Jiang J (2008) Computer simulation for adsorption of CO2, N2 and flue gas in a mimetic MCM-41. J Phys Chem C 112:11295–11300. https://doi.org/10.1021/jp803428n
Liu B, Smit B (2010) Molecular simulation studies of separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J Phys Chem C 114:8515–8522. https://doi.org/10.1021/jp101531m
Smit B, Maesen TLM (2008) Molecular simulations of zeolites: adsorption, diffusion, and shape selectivity. Chem Rev 108:4125–4184. https://doi.org/10.1021/cr8002642
Sarkisov L, Harrison A (2011) Computational structure characterisation tools in application to ordered and disordered porous materials. Mol Simulat 37:1248–1257. https://doi.org/10.1080/08927022.2011.592832
Funding
This work was supported by the National Natural Science Foundation of China (No. 52174058 and No. 51804045), the Key Research and Development Program of Jiangsu Province (Industry Foresight and Common Key Technology, No. BE2018065), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX21_2869, No. KYCX21_2790, and No. SJCX21_1208).
Author information
Authors and Affiliations
Contributions
Conceptualization: Weiqiu Huang and Weihua Chen. Funding acquisition: Weiqiu Huang, Lipei Fu and Xufei Li. Methodology: Weiqiu Huang, Lipei Fu and Xufei Li. Writing—original draft: Weihua Chen and Xinya Wang. Formal analysis: Yongyin Zheng, Xinya Wang and Weihua Chen. Software and data curation: Yilong Zhang. Supervision: Jiahui Zhu and Bing Zhu.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Huang, W., Fu, L. et al. Simulation study on the effect of pore structure and surface curvature of activated carbon on the adsorption and separation performance of CO2/N2. J Nanopart Res 24, 185 (2022). https://doi.org/10.1007/s11051-022-05569-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05569-2