Abstract
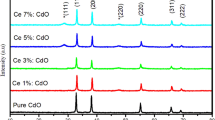
Molten salts synthesis (MSS) is a simple and potential technique for ceria nanoparticles (NPs) production, but the relevant effects of salt medium and cerium precursor on the synthesized ceria NPs need further attention. In the present work, we investigated the influences of frequently employed KCl–LiCl and KOH–NaOH molten flux and typical inorganic cerium precursors on the characterizations and photocatalytic property of ceria NPs. Results show that the CeO2 NPs obtained in molten KOH–NaOH flux have smaller particle/crystal sizes but more serious agglomeration than that from KCl–LiCl flux. UV–Vis DRS spectra reveal that the ceria synthesized in molten hydroxides from the cerium nitrate precursor has the narrowest band gap of 2.87 eV in studied ceria samples. The ceria NPs obtained from KCl–LiCl flux have wider band gaps of 3.15–3.29 eV and faster recombination rates of photo-generated e− and h+. It was found that the ceria NPs obtained in molten KCl–LiCl salt show better photocatalytic activities for methylene blue (MB) degradation and may be due to the uniform size distribution and well-dispersed particles.










Similar content being viewed by others
References
Abitbol T, Ahniyaz A, Álvarez-Asencio R, Fall A, Swerin A (2020) Nanocellulose-based hybrid materials for UV blocking and mechanically robust barriers. ACS Appl Bio Mater 3(4):2245–2254
Ackermann S, Sauvin L, Castiglioni R, Rupp JL, Scheffe JR, Steinfeld A (2015) Kinetics of CO2 reduction over nonstoichiometric ceria. J Phys Chem C 119(29):16452–16461
Ahrens T J. Global earth physics: a handbook of physical constants: American Geophysical Union, 1995.
Alagar M (2018) Synthesis, Structural and optical behavior of cerium oxide nanoparticles by co-precipitation method. Int J Sci Res Sci Technol 4(5):962
Amoresi RAC, Oliveira RC, Marana NL, de Almeida PB, Prata PS, Zaghete MA, Longo E, Sambrano JR, Simões AZ (2019) CeO2 Nanoparticle morphologies and their corresponding crystalline planes for the photocatalytic degradation of organic pollutants. ACS Appl Nano Mater 2(10):6513–6526
Bakkiyaraj R, Balakrishnan M, Bharath G, Ponpandian N (2017) Facile synthesis, structural characterization, photocatalytic and antimicrobial activities of Zr doped CeO2 nanoparticles. J Alloys Compd 724:555–564
Bondioli F, Corradi AB, Leonelli C (1999) Nanosized CeO2 powders obtained by flux method. Mater Res Bull 34(14/15):2159
Chen Y, Cai W, Wang W, Chen A (2019) Preparation, characterization, and application of dendritic silica-supported samarium-doped ceria nanoparticles in ultra-precision polishing for silica films. J Nanoparticle Res 21(11):226
Chueh WC, Falter C, Abbott M, Scipio D, Furler P, Haile SM, Steinfeld A (2010) High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330(6012):1797–1801
El Rouby WM, Farghali AA, Hamdedein A (2016) Microwave synthesis of pure and doped cerium (IV) oxide (CeO2) nanoparticles for methylene blue degradation. Water Sci Technol 74(10):2325–2336
El-Enein SAA, Ali AM, Abdel-Monem YK, Senna MH, Madkour M (2019) Novel lanthanide(III) 4-methylbenzoylhydrazide complexes as precursors for lanthanide oxide nanophotocatalysts. RSC Adv 9(72):42010–42019
Feng X, Sayle DC, Wang ZL, Paras MS, Santora B, Sutorik AC, Sayle TX, Yang Y, Ding Y, Wang X (2006) Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 312(5779):1504–1508
Goguet A, Meunier FC, Tibiletti D, Breen JP, Burch R (2004) Spectrokinetic investigation of reverse water-gas-shift reaction intermediates over a Pt/CeO2 catalyst. J Phys Chem B 108(52):20240–20246
Gokon N, Suda T, Kodama T (2015) Oxygen and hydrogen productivities and repeatable reactivity of 30-mol%-Fe-, Co-, Ni-, Mn-doped CeO2−δ for thermochemical two-step water-splitting cycle. Energy 90:1280–1289
Habib IY, Burhan J, Jaladi F, Lim CM, Usman A, Kumara NTRN, Tsang SCE, Mahadi AH (2020) Effect of Cr doping in CeO2 nanostructures on photocatalysis and H2O2 assisted methylene blue dye degradation. Catal Today. https://doi.org/10.1016/j.cattod.2020.04.008
Huang Y, Yan C-F, Guo C-Q, Huang S-L (2015) Enhanced photoreduction activity of carbon dioxide over Co3O4/CeO2 catalysts under visible light irradiation. Int J Photoenergy 2015:30808
Jasinski P, Suzuki T, Anderson HU (2003) Nanocrystalline undoped ceria oxygen sensor. Sensors and Actuators B: Chem 95(1):73–77
Jha A, Jeong D-W, Jang W-J, Lee Y-L, Roh H-S (2015) Hydrogen production from water–gas shift reaction over Ni–Cu–CeO2 oxide catalyst: the effect of preparation methods. Int J Hydrog Energy 40(30):9209–9216
Kašpar J, Fornasiero P, Graziani M (1999) Use of CeO2-based oxides in the three-way catalysis. Catalysis Today. 50(2):285–298
Kesarla MK, Fuentez-Torres MO, Alcudia-Ramos MA, Ortiz-Chi F, Espinosa-González CG, Aleman M, Torres-Torres JG, Godavarthi S (2019) Synthesis of g-C3N4/N-doped CeO2 composite for photocatalytic degradation of an herbicide. J Mater Res Technol 8(2):1628–1635
Khadar YAS, Balamurugan A, Devarajan VP, Subramanian R (2017) Hydrothermal synthesis of gadolinium (Gd) doped cerium oxide (CeO2) nanoparticles: characterization and antibacterial activity. Orient J Chem 33(5):2405–2411
Lan YP, Hong YS, Mohassab Y, Liu Q, Xu B (2017) Properties of stable nonstoichiometric nanoceria produced by thermal plasma. J Nanoparticle Res 19(8):281
Lan Y-P, Sohn HY (2018) Nanoceria synthesis in molten KOH-NaOH mixture: characterization and oxygen vacancy formation. Ceram Int 44(4):3847–3855
Lan YP, Sohn HY, Mohassab Y, Liu Q, Xu B (2017) Nanoceria synthesis in the KCl-LiCl salt system: crystal formation and properties. J Am Ceram Soc 100(5):1863–1875
Lan Y-P, Sohn HY, Murali A, Li J, Chen C (2018) The formation and growth of CeOCl crystals in a molten KCl-LiCl flux. Appl Phys A 124(10):702
Latha P, Prakash K, Karuthapandian S (2017) Enhanced visible light photocatalytic activity of CeO2/alumina nanocomposite: synthesized via facile mixing-calcination method for dye degradation. Adv Powder Technol 28(11):2903–2913
Li Y, Li W, Liu F, Li M, Qi X, Xue M, Wang Y, Han F (2020) Construction of CeO2/TiO2 heterojunctions immobilized on activated carbon fiber and its synergetic effect between adsorption and photodegradation for toluene removal. J Nanoparticle Res 22(5):122
Li L, Zhu B, Zhang J, Yan C, Wu Y (2018) Electrical properties of nanocube CeO2 in advanced solid oxide fuel cells. I J Hydrog Energy 43(28):12909–12916
Liu X, Fechler N, Antonietti M (2013) Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem Soc Rev 42(21):8237–8265
Lu L, Dai G, Yan L, Wang L, Wang L, Wang Z, Wei K (2020) In-situ low-temperature sol–gel growth of nano-cerium oxide ternary composite films for ultraviolet blocking. Opt Mater 101:109724
Magudieshwaran R, Ishii J, Raja KCN, Terashima C, Venkatachalam R, Fujishima A, Pitchaimuthu S (2019) Green and chemical synthesized CeO2 nanoparticles for photocatalytic indoor air pollutant degradation. Mater Lett 239:40–44
Miao H, Huang G-F, Liu J-H, Zhou B-X, Pan A, Huang W-Q, Huang G-F (2016) Origin of enhanced photocatalytic activity of F-doped CeO2 nanocubes. Appl Surf Sci 370:427–432
Oosthuizen DN, Motaung DE, Swart HC (2020) Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl Surf Sci 505:144356
Phuruangrat A, Thongtem S, Thongtem T (2017) Microwave-assisted hydrothermal synthesis and characterization of CeO2 nanowires for using as a photocatalytic material. Mater Lett 196:61–63
Pudukudy M, Jia Q, Yuan J, Megala S, Rajendran R, Shan S (2020) Influence of CeO2 loading on the structural, textural, optical and photocatalytic properties of single-pot sol–gel derived ultrafine CeO2/TiO2 nanocomposites for the efficient degradation of tetracycline under visible light irradiation. Mater Sci Semicond Process 108:104891
Qi L, Yu Q, Dai Y, Tang C, Liu L, Zhang H, Gao F, Dong L, Chen Y (2012) Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl Catal B: Environ 119-120:308–320
Rajan AR, Vilas V, Rajan A, John A, Philip D (2020) Synthesis of nanostructured CeO2 by chemical and biogenic methods: optical properties and bioactivity. Ceram Int 46(9):14048–14055
Reed K, Cormack A, Kulkarni A, Mayton M, Sayle D, Klaessig F, Stadler B (2014) Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom? Environ Sci: Nano 1(5):390–405
Roh H-S, Potdar HS, Jeong D-W, Kim K-S, Shim J-O, Jang W-J, Koo KY, Yoon WL (2012) Synthesis of highly active nano-sized (1wt.% Pt/CeO2) catalyst for water gas shift reaction in medium temperature application. Catal Today 185(1):113
Saravanan R, Joicy S, Gupta VK, Narayanan V, Stephen A (2013) Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts. Mater Sci Eng C Mater Biol Appl 33(8):4725–4731
Umar A, Almas T, Ibrahim AA, Kumar R, AlAssiri MS, Baskoutas S, Akhtar MS (2020) An efficient chemical sensor based on CeO2 nanoparticles for the detection of acetylacetone chemical. J Electroanal Chem 864:114089
Xia X, Lan Y, Li J, Chen C, Xu B, Luo X, Mao X (2020) Facile synthesis of nanoceria by a molten hydroxide method and its photocatalytic properties. J Rare Earths 38(9):951–960
Yang X, Liu Y, Li J, Zhang Y (2019) Effects of calcination temperature on morphology and structure of CeO2 nanofibers and their photocatalytic activity. Mater Lett 241:76–79
Yang J, Lukashuk L, Li H, Fottinger K, Rupprechter G, Schubert U (2014) High surface area ceria for CO oxidation prepared from cerium t-butoxide by combined sol-gel and solvothermal processing. Catal Lett 144(3):403–412
Yang C, Yang J, Duan X, Hu G, Liu Q, Ren S, Li J, Kong M (2020) Roles of photo-generated holes and oxygen vacancies in enhancing photocatalytic performance over CeO2 prepared by molten salt method. Adv Powder Technol 31(9):4072–4081
Zeng M, Li Y, Mao M, Bai J, Ren L, Zhao X (2015) Synergetic effect between photocatalysis on TiO2 and thermocatalysis on CeO2 for gas-phase oxidation of benzene on TiO2/CeO2 nanocomposites. ACS Catal 5(6):3278–3286
Zhang J, Yang J, Wang J, Ding H, Liu Q, Schubert U, Rui Y, Xu J (2017) Surface oxygen vacancies dominated CeO2 as efficient catalyst for imine synthesis: influences of different cerium precursors. Mol Catal 443:131–138
Zhang G, Yang Z, Zhang W, Wang Y (2017) Nanosized Mo-doped CeO2 enhances the electrocatalytic properties of the Pt anode catalyst in direct methanol fuel cells. J Mater Chem A 5(4):1481–1487
Zhao X, Wu P, Lu D, Fang P, Liu M, Qian Z (2017) Synergistic effect of Fe2O3 and CeO2 co-modified titanate nanosheet heterojunction on enhanced photocatalytic degradation. J Nanoparticle Res 19(9):325
Acknowledgments
The financial support from National Natural Science Foundation of China under Grant No. 51804088, the Talents & Platform Funding from Science & Technology Department of Guizhou Province, China, under the Grant No. [2017]5788 and [2018]5781, the Basic Research Program from Science & Technology Department of Guizhou Province [2020]1Y219 and [2019]1082, and the Cultivation Funding of No. 2019 and Doctor Funding of No. (2017)04 supported by Guizhou University are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, X., Xia, X., Ning, D. et al. Characterizations and photocatalytic activity of ceria nanoparticles synthesized in KCl–LiCl/KOH–NaOH molten flux from different precursors. J Nanopart Res 23, 69 (2021). https://doi.org/10.1007/s11051-021-05172-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-021-05172-x