Abstract
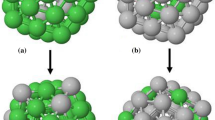
Molecular dynamics simulation was employed to understand the thermodynamic behavior of cuboctahedron (cub) and icosahedron (ico) nanoparticles with 2–20 number of full shells. The original embedded atom method (EAM) was compared to the more recent highly optimized version as inter-atomic potential. The thermal stability of clusters were probed using potential energy and specific heat capacity as well as structure analysis by radial distribution function (G(r)) and common neighbor analysis (CNA), simultaneously, to make a comprehensive picture of the solid-state and melting transitions. The result shows ico is the only stable shape of small clusters (Pd55–Pd309 using original EAM and Pd55 using optimized version) those are melting uniformly due to their small diameter. An exception is cub Pd309 modeled via optimized EAM that transforms to ico at elevated temperatures. A similar cub to ico transition was predicted by original EAM for Pd923–Pd2075 clusters, while for the larger clusters both cub and ico are stable up to the melting point. As detected by \(G(r)\) and CNA, moderate and large cub clusters were showing surface melting by nucleation of the liquid phase at (100) planes and growth of liquid phase at the surface before inward growth. While diagonal (one corner to another) melting was dominating over ico clusters owing to their partitioned structure, which retarded the growth of the liquid phase. The large ico clusters, using optimized EAM, presented a combination of surface and diagonal melting due to the simultaneous diagonal melting started from different corners. Finally, the melting temperature as well as latent heat of fusion were calculated and compared with the available models and previous studies, which showed, unlike the present result, the models failed to predict size-dependent motif cross-over.








Similar content being viewed by others
Notes
version 14 Apr 2013 available at http://lammps.sandia.gov
Version 2.7.1 available at http://ovito.org/
References
Alavi S, Thompson DL (2006) Molecular dynamics simulations of the melting of aluminum nanoparticles. The J Phys Chem A 110(4):1518–1523
Alemany MMG, Diéguez O, Rey C, Gallego LJ (1999) Molecular-dynamics study of the dynamic properties of fcc transition and simple metals in the liquid phase using the second-moment approximation to the tight-binding method. Physical Review B: Condensed Matter 60(13):9208–9211. https://doi.org/10.1103/PhysRevB.60.9208
Allen MP, Tildesley DJ (1989) Computer simulation of liquids. Oxford university press, Oxford
Ashcroft NW, Mermin ND (1976) Solid state physics holt. Rinehart and Winston, New York
Attarian Shandiz M, Safaei A (2008) Melting entropy and enthalpy of metallic nanoparticles. Mater Lett 62(24):3954–3956
Baletto F, Mottet C, Ferrando R (2000) Reentrant morphology transition in the growth of free silver nanoclusters. Phys Rev Lett 84(24):5544
Baletto F, Mottet C, Ferrando R (2001) Microscopic mechanisms of the growth of metastable silver icosahedra. Phys Rev B 63(15):155,408
Baletto F, Ferrando R, Fortunelli A, Montalenti F, Mottet C (2002) Crossover among structural motifs in transition and noble-metal clusters. The J Chem Phys 116(9):3856–3863
Bertoldi DS, Millán EN, Guillermet AF (2017) Thermodynamics of the melting process in au nano-clusters: phenomenology, energy, entropy and quasi-chemical modeling. J Phys Chem Solids 111:286–293
Chen T, Zhang Y, Xu W (2016) Size-dependent catalytic kinetics and dynamics of Pd nanocubes: a single-particle study. Phys Chem Chem Phys 18(32):22,494–22,502
Dinsdale AT (1991) SGTE Data for pure elements. Calphad 15(4):317–425
Faken D, Jónsson H (1994) Systematic analysis of local atomic structure combined with 3d computer graphics. Comput Mater Sci 2(2):279–286
Foiles S, Baskes M, Daw M (1986) Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys Rev B 33(12):7983
Fu Q, Zhu J, Xue Y, Cui Z (2017) Size- and shape-dependent melting enthalpy and entropy of nanoparticles. J Mater Sci 52(4):1911–1918. https://doi.org/10.1007/s10853-016-0480-9
Goldstein AN, Echer CM, Alivisatos AP (1992) Melting in semiconductor nanocrystals. Science 256(5062):1425–1427
Iida T, Guthrie RIL (1988) The physical properties of liquid metals. Clarendon Press, Oxford
Jiang Q, Shi HX, Zhao M (1999) Melting thermodynamics of organic nanocrystals. The J Chem Phys 111(5):2176–2180
Jiang Q, Yang CC, Li JC (2002) Melting enthalpy depression of nanocrystals. Mater Lett 56 (6):1019–1021
José-Yacamán M, Marín-Almazo M, Ascencio JA (2001) High resolution TEM studies on palladium nanoparticles. J Mol Catal A Chem 173(1):61–74
Kateb M, Dehghani K (2012) Comparison of fracture behavior of sharp with blunt crack tip in nanocrystalline materials by molecular dynamics simulation. Int J Mod Phys: Conference Series 5:410–417
Kraftmakher YA (1986) Equilibrium concentration of vacancies in metals. In: Seeger A, Schumacher D, Schilling W, Diehl J (eds) Vacancies and interstitials in metals: international conference proceeding, North Holland, Amsterdam, pp 59, held in jülich, Germany September pp 23-28
Lee YJ, Lee EK, Kim S, Nieminen RM (2001) Effect of potential energy distribution on the melting of clusters. Phys Rev Lett 86(6):999
Liang T, Zhou D, Wu Z, Shi P (2017) Size-dependent melting modes and behaviors of Ag nanoparticles: a molecular dynamics study. Nanotechnology 28(48):485,704
Lindemann FA (1910) The calculation of molecular vibration frequencies. Physikalische Zeitschrift 11:609–612
Miao L, Bhethanabotla VR, Joseph B (2005) Melting of Pd clusters and nanowires: a comparison study using molecular dynamics simulation. Phys Rev B 72(13):134,109
Pan Y, Huang S, Liu Z, Wang W (2005) Molecular dynamics simulation of shell-symmetric Pd nanoclusters. Mol Simul 31(14-15):1057–1061
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117 (1):1–19
Plimpton SJ, Thompson AP (2012) Computational aspects of many-body potentials. MRS Bull 37(5):513–521
Poole CP Jr, Owens FJ (2003) Introduction to nanotechnology. Wiley, New Jersey
Qi W (2016) Nanoscopic thermodynamics. Acc Chem Res 49(9):1587–1595
Qi Y, Çagin T, Johnson WL, Goddard WA III (2001) Melting and crystallization in Ni nanoclusters: the mesoscale regime. The J Chem Phys 115(1):385–394
Rangel E, Sansores E, Vallejo E, Hernández-Hernández A, López-Pérez P (2016) Study of the interplay between N-graphene defects and small Pd clusters for enhanced hydrogen storage via a spill-over mechanism. Phys Chem Chem Phys 18(48):33, 158–33,170
Rao CN, Rao KK (1964) Effect of temperature on the lattice parameters of some silver-palladium alloys. Can J Phys 42(7):1336–1342
Rossi G, Ferrando R (2007) Freezing of gold nanoclusters into poly-decahedral structures. Nanotechnology 18(22):225,706
Safaei A (2010) The effect of the averaged structural and energetic features on the cohesive energy of nanocrystals. J Nanoparticle Res 12(3):759–776
Safaei A, Shandiz MA, Sanjabi S, Barber ZH (2008) Modeling the melting temperature of nanoparticles by an analytical approach. The J Phys Chem C 112(1):99–105. https://doi.org/10.1021/jp0744681
Schebarchov D, Hendy S (2006) Solid-liquid phase coexistence and structural transitions in palladium clusters. Physical Review B 73(12):121,402
Schmidt M, Kusche R, von Issendorff B, Haberland H (1998) Irregular variations in the melting point of size-selected atomic clusters. Nature 393(6682):238–240
Sheng HW, Kramer MJ, Cadien A, Fujita T, Chen MW (2011) Highly optimized embedded-atom-method potentials for fourteen fcc metals. Phys Rev B 83(13):134,118. https://doi.org/10.1103/PhysRevB.83.134118
Shim JH, Lee BJ, Cho YW (2002) Thermal stability of unsupported gold nanoparticle: a molecular dynamics study. Surf Sci 512(3):262–268
Steinhardt PJ, Nelson DR, Ronchetti M (1983) Bond-orientational order in liquids and glasses. Phys Rev B 28(2):784
Stukowski A (2009) Visualization and analysis of atomistic simulation data with OVITO–the open visualization tool. Model Simul Mater Sci Eng 18(1):015,012
Tsuzuki H, Branicio PS, Rino JP (2007) Structural characterization of deformed crystals by analysis of common atomic neighborhood. Comput Phys Commun 177(6):518–523
Tyson WR, Miller WA (1977) Surface free energies of solid metals: estimation from liquid surface tension measurements. Surf Sci 62(1):267–276
Vanselow R, Howe RF (1988) Chemistry and physics of solid surfaces VII, vol 10. Springer, Berlin
Verlet L (1967) Computer “experiments” on classical fluids. I. Thermodynamical properties of lennard-Jones molecules. Phys Rev 159(1):98
Wang W, Xu J, Zhao Y, Qi G, Wang Q, Wang C, Li J, Deng F (2017) Facet dependent pairwise addition of hydrogen over Pd nanocrystal catalysts revealed via NMR using para-hydrogen-induced polarization. Phys Chem Chem Phys 19(14):9349–9353
Waseda Y (1980) The structure of non-crystalline materials: liquids and amorphous solids. McGraw-Hill International Book Co., New York
Westergren J, Nordholm S (2003) Melting of palladium clusters–density of states determination by Monte Carlo simulation. Chem Phys 290(2):189–209
Zhang H, Douglas JF (2013) Glassy interfacial dynamics of Ni nanoparticles: part I colored noise, dynamic heterogeneity and collective atomic motion. Soft matter 9(4):1254–1265
Zhang M, Efremov MY, Schiettekatte F, Olson EA, Kwan AT, Lai SL, Wisleder T, Greene JE, Allen LH (2000) Size-dependent melting point depression of nanostructures: nanocalorimetric measurements. Phys Rev B 62(15): 10,548
Zhang Y, Wen YH, Zhu ZZ, Sun SG (2010) Structure and stability of fe nanocrystals: an atomistic study. The J Phys Chem C 114(44):18,841–18,846
Zhao SJ, Wang SQ, Cheng DY, Ye HQ (2001) Three distinctive melting mechanisms in isolated nanoparticles. The J Phys Chem B 105(51):12,857–12,860
Zhivonitko VV, Skovpin IV, Crespo-Quesada M, Kiwi-Minsker L, Koptyug IV (2016) Acetylene oligomerization over Pd nanoparticles with controlled shape: a parahydrogen-induced polarization study. The J Phys Chem C 120(9):4945–4953. https://doi.org/10.1021/acs.jpcc.5b12391
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(MP4 5.49 MB)
(MP4 2.89 MB)
(MP4 5.45 MB)
Rights and permissions
About this article
Cite this article
Kateb, M., Azadeh, M., Marashi, P. et al. Size and shape-dependent melting mechanism of Pd nanoparticles. J Nanopart Res 20, 251 (2018). https://doi.org/10.1007/s11051-018-4355-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4355-7