Abstract
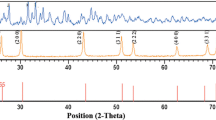
A novel synthetic approach for the preparation of PbS micro-nanostructures with different morphologies has been reported. PbS micro-nanostructures with various morphologies such as stars, dendrites, hexapods, and cubes were synthesized by thermal decomposition of lead acetate and thiourea in ethylene glycol at 120 °C, in air, in the absence of any surfactant. The PbS micro-nanostructures were characterized using different analytical techniques such as X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, diffuse reflectance spectroscopy, and photoluminescence spectroscopy. The effect of different synthetic parameters such as [Pb2+:S2−] ratio, decomposition temperature, time, and source of sulfur on the morphologies of PbS was investigated and the mechanism for the formation of micro-nanostructures with different morphologies has also been proposed.












Similar content being viewed by others
References
Apte SK, Garaje SN, Mane GP, Vinu A, Naik SD, Amalnerkar DP, Kale BB (2011) A facile template-free approach for the large-scale solid-phase synthesis of CdS nanostructures and their excellent photocatalytic performance. Small 7(7):957–964. doi:10.1002/smll.201002130
Bakshi MS, Thakur P, Sachar S, Kaur G, Banipal TS, Possmayer F, Petersen NO (2007) Aqueous phase surfactant selective shape controlled synthesis of lead sulfide nanocrystals. J Phys Chem C 111(49):18087–18098. doi:10.1021/jp075477c
Bashouti M, Lifshitz E (2008) PbS sub-micrometer structures with anisotropic shape: ribbons, wires, octapods, and hollowed cubes. Inorg Chem 47(2):678–682. doi:10.1021/ic700706a
Borhade AV, Uphade BK (2012) A comparative study on characterization and photocatalytic activities of PbS and Co doped PbS nanoparticles. Chalcogenide Lett 9(7):299–306
Bu J, Nie C, Liang J, Sun L, Xie Z, Wu Q, Lin C (2011) Synthesis of single-crystal PbS nanorods via a simple hydrothermal process using PEO–PPO–PEO triblock copolymer as a structure-directing agent. Nanotechnology 22(12):125602/1–125602/7. doi:10.1088/0957-4484/22/12/125602
Buckner SW, Konold RL, Jelliss PA (2004) Luminescence quenching in PbS nanoparticles. Chem Phys Lett 394(4–6):400–404. doi:10.1016/j.cplett.2004.06.138
Devi PI, Sivabharathy M, Ramachandran K (2013) Enhancement of dielectric constant in PVDF polymer using dendrite-shaped PbS nanostructures. Optik 124(19):3872–3875. doi:10.1016/j.ijleo.2012.11.038
Ding B, Shi M, Chen F, Zhou R, Deng M, Wang M, Chen H (2009) Shape-controlled syntheses of PbS submicro-/nano-crystals via hydrothermal method. J Cryst Growth 311(6):1533–1538. doi:10.1016/j.jcrysgro.2009.01.086
Hansen JA, Mukhopadhyay R, Hansen JO, Gothelf KV (2006) Femtomolar electrochemical detection of DNA targets using metal sulfide nanoparticles. J Am Chem Soc 128(12):3860–3861. doi:10.1021/ja0574116
Hou Y, Kondoh H, Ohta T (2009) PbS cubes with pyramidal pits: an example of etching growth. Cryst Growth Des 9(7):3119–3123. doi:10.1021/cg801013t
Hu K, Liu P, Ye S, Zhang S (2009) Ultrasensitive electrochemical detection of DNA based on PbS nanoparticle tags and nanoporous gold electrode. Biosens Bioelectron 24(10):3113–3119. doi:10.1016/j.bios.2009.04.001
Huang T, Zhao Q, Xiao J, Qi L (2010) Controllable self-assembly of PbS nanostars into ordered structures: close-packed arrays and patterned arrays. ACS Nano 4(8):4707–4716. doi:10.1021/nn101272y
Liu S, Xiong S, Bao K, Cao J, Qian Y (2009) Shape-controlled preparation of PbS with various dendritic hierarchical structures with the assistance of l-methionine. J Phys Chem C 113(30):13002–13007. doi:10.1021/jp8104437
Liu M, Leng M, Liu D, Chen F, Li C, Wang C (2014) Local supersaturation dictated branching and faceting of submicrometer PbS particles with cubic growth habit. Inorg Chem 53(21):11484–11491. doi:10.1021/ic501368y
Lovette MA, Browning AR, Griffin DW, Sizemore JP, Snyder RC, Doherty MF (2008) Crystal shape engineering. Ind Eng Chem Res 47(24):9812–9833. doi:10.1021/ie800900f
Ma Y, Qi L, Ma J, Cheng H (2004) Hierarchical, star-shaped PbS crystals formed by a simple solution route. Cryst Growth Des 4(2):351–354. doi:10.1021/cg034174e
Malgras V, Nattestad A, Yamauchi Y, Dou SX, Kim JH (2015) The effect of surface passivation on the structure of sulphur-rich PbS colloidal quantum dots for photovoltaic application. Nanoscale 7(13):5706–5711. doi:10.1039/c4nr07006b
Mandal T, Piburn G, Stavila V, Rusakova I, Ould-Ely T, Colson AC, Whitmire KH (2011) New mixed ligand single-source precursors for PbS nanoparticles and their solvothermal decomposition to anisotropic nano- and microstructures. Chem Mater 23(18):4158–4169. doi:10.1021/cm201064c
McDonald SA, Konstantatos G, Zhang SG, Cyr PW, Klem EJD, Levina L, Sargent EH (2005) Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nat Mater 4(2):138–142. doi:10.1038/nmat1299
McPhail MR, Weiss EA (2014) Role of organosulfur compounds in the growth and final surface chemistry of PbS quantum dots. Chem Mater 26(11):3377–3384. doi:10.1021/cm4040819
Ni Y, Wang X, Hong J (2012) Fast reflux synthesis of multi-armed PbS dendrites, influencing factors and optical properties. RSC Adv 2(2):546–551. doi:10.1039/c1ra00769f
Peng Z, Jiang Y, Song Y, Wang C, Zhang H (2008) Morphology control of nanoscale PbS particles in a polyol process. Chem Mater 20(9):3153–3162. doi:10.1021/cm703707v
Phuruangrat A, Thongtem T, Thongtem S (2008) Characterization of PbS with different morphologies produced using a cyclic microwave radiation. Appl Surf Sci 254(23):7553–7558. doi:10.1016/j.apsusc.2008.01.059
Phuruangrat A, Thongtem T, Kuntalue B, Thongtem S (2012) Characterization of cubic and star-shaped dendritic PbS structures synthesized by a solvothermal method. Mater Lett 81:55–58. doi:10.1016/j.matlet.2012.04.129
Podsiadlo P, Lee B, Prakapenka VB, Krylova GV, Schaller RD, Demortiere A, Shevchenko EV (2011) High-pressure structural stability and elasticity of supercrystals self-assembled from nanocrystals. Nano Lett 11(2):579–588. doi:10.1021/nl103587u
Quan Z, Li C, Zhang X, Yang J, Yang P, Zhang C, Lin J (2008) Polyol-mediated synthesis of PbS crystals: shape evolution and growth mechanism. Cryst Growth Des 8(7):2384–2392. doi:10.1021/cg701236v
Querejeta-Fernandez A, Hernandez-Garrido JC, Yang H, Zhou Y, Varela A, Parras M, Kotov NA (2012) Unknown aspects of self-assembly of PbS microscale superstructures. ACS Nano 6(5):3800–3812. doi:10.1021/nn300890s
Saraidarov T, Reisfeld R, Sashchiuk A, Lifshitz E (2007) Synthesis and characterization of PbS nanorods and nanowires. Physica E 37(1–2):173–177. doi:10.1016/j.physe.2006.07.015
Shao S, Zhang G, Zhou H, Sun P, Yuan Z, Li B, Chen T (2007) Morphological evolution of PbS crystals under the control of l-lysine at different pH values: the ionization effect of the amino acid. Solid State Sci 9(8):725–731. doi:10.1016/j.solidstatesciences.2007.06.002
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL (2015) In: Brennan D (ed) Spectrometric identification of organic compounds, 8th edn. Wiley, New York
Snyder RC, Doherty MF (2007) Faceted crystal shape evolution during dissolution or growth. AIChE J 53(5):1337–1348. doi:10.1002/aic.11132
Souici AH, Keghouche N, Delaire JA, Remita H, Etcheberry A, Mostafavi M (2009) Structural and optical properties of PbS nanoparticles synthesized by the radiolytic method. J Phys Chem C 113(19):8050–8057. doi:10.1021/jp811133b
Stuart B (2004) Infrared spectroscopy: fundamentals and applications. Wiley, Chichester
Wang D, Yu DB, Shao MW, Liu XM, Yu WC, Qian YT (2003) Dendritic growth of PbS crystals with different morphologies. J Cryst Growth 257(3–4):384–389. doi:10.1016/s0022-0248(03)01470-2
Wang N, Cao X, Guo L, Yang S, Wu Z (2008) Facile synthesis of PbS truncated octahedron crystals with high symmetry and their large-scale assembly into regular patterns by a simple solution route. ACS Nano 2(2):184–190. doi:10.1021/nn7000855
Wang Y, Dai Q, Yang X, Zou B, Li D, Liu B, Zou G (2011) A facile approach to PbS nanoflowers and their shape-tunable single crystal hollow nanostructures: morphology evolution. CrystEngComm 13(1):199–203. doi:10.1039/c004459h
Wang Y, Yang X, Xiao G, Zhou B, Liu B, Zou G, Zou B (2013) Shape-controlled synthesis of PbS nanostructures from −20 to 240 °C: the competitive process between growth kinetics and thermodynamics. CrystEngComm 15(27):5496–5505. doi:10.1039/c3ce40337h
Wu W, He Y, Wu Y, Wu T (2011) Self-template synthesis of PbS nanodendrites and its photocatalytic performance. J Alloy Compd 509(38):9356–9362. doi:10.1016/j.jallcom.2011.07.036
Xiang J, Cao H, Wu Q, Zhang S, Zhang X (2008) l-Cysteine-assisted self-assembly of complex PbS structures. Cryst Growth Des 8(11):3935–3940. doi:10.1021/cg7007842
Yadav SK, Jeevanandam P (2015) Synthesis of PbS–Al2O3 nanocomposites by sol–gel process and studies on their optical properties. Opt Mater 46:209–215. doi:10.1016/j.optmat.2015.04.020
Zhang W, Yang Q, Xu L, Yu W, Qian Y (2005) Growth of PbS crystals from nanocubes to eight-horn-shaped dendrites through a complex synthetic route. Mater Lett 59(27):3383–3388. doi:10.1016/j.matlet.2004.09.065
Zhao N, Qi L (2006) Low-temperature synthesis of star-shaped PbS nanocrystals in aqueous solutions of mixed cationic/anionic surfactants. Adv Mater 18(3):359–362. doi:10.1002/adma.200501756
Zhao XS, Xu SY, Liang LY, Li T, Cauchi S (2007) Luminescent stability of water-soluble PbS nanoparticles. J Mater Sci 42(12):4265–4269. doi:10.1007/s10853-006-0679-2
Zhou G, Lu M, Xiu Z, Wang S, Zhang H, Zhou Y, Wang S (2006) Controlled synthesis of high-quality PbS star-shaped dendrites, multipods, truncated nanocubes, and nanocubes and their shape evolution process. J Phys Chem B 110(13):6543–6548. doi:10.1021/jp0549881
Acknowledgments
The award of Junior/Senior Research Fellowship (JRF/SRF) to Ms. Rama Gaur by the Council of Scientific and Industrial Research, Government of India, is gratefully acknowledged. Thanks are also due to the Institute Instrumentation Centre, IIT Roorkee, for providing some of the facilities used in the present study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaur, R., Jeevanandam, P. PbS micro-nanostructures with controlled morphologies by a novel thermal decomposition approach. J Nanopart Res 18, 80 (2016). https://doi.org/10.1007/s11051-016-3382-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3382-5