Abstract
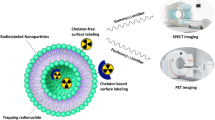
Developing nanoparticulate delivery systems that will allow easy movement and localization of a drug to the target tissue and provide more controlled release of the drug in vivo is a challenge in nanomedicine. The aim of this study was to evaluate the biodistribution of poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles containing samarium-153 oxide ([153Sm]Sm2O3) in vivo to prove that orally administered nanoparticles alter the biodistribution of a drug. These were then activated in a nuclear reactor to produce radioactive 153Sm-loaded-PLGA nanoparticles. The nanoparticles were characterized for size, zeta potential, and morphology. The nanoparticles were orally and intravenously (IV) administered to rats in order to trace their uptake through imaging and biodistribution studies. The 153Sm-loaded-PLGA nanoparticles had an average size of 281 ± 6.3 nm and a PDI average of 0.22. The zeta potential ranged between 5 and 20 mV. The [153Sm]Sm2O3 loaded PLGA nanoparticles, orally administered were distributed to most organs at low levels, indicating that there was absorption of nanoparticles. While the IV injected [153Sm]Sm2O3-loaded PLGA nanoparticles exhibited the highest localization of nanoparticles in the spleen (8.63 %ID/g) and liver (3.07 %ID/g), confirming that nanoparticles are rapidly removed from the blood by the RES, leading to rapid uptake in the liver and spleen. From the biodistribution data obtained, it is clear that polymeric nanoscale delivery systems would be suitable for improving permeability and thus the bioavailability of therapeutic compounds.
Graphical Abstract






Similar content being viewed by others
References
Ali MM, Yoo B, Pagel MD (2009) Tracking the relative in vivo pharmacokinetics of nanoparticles with PARACEST MRI. Mol Pharm 6(5):1409–1416
Caruthers SD, Wickline SA, Lanza GM (2007) Nanotechnological applications in medicine. Curr Opin Biotechnol 18(1):26–30
Koo OM, Rubinstein I, Onyuksel H (2005) Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine 1:193–212
Lambrecht A, Ubrich N, Perez MH, Lehr CM, Hoffman J, Maincent P (1999) Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm 184(1):97–105
Mohammed AK, Reineke JJ (2013) Quantitative detection of PLGA nanoparticles degradation in tissues following intravenous administration. Mol Pharm 10(6):2183–2189
Orive G, Hernandez RM, Gascon AR, Dominguez-Gil A, Pedra ZJ (2003) Drug delivery in biotechnology: present and future. Curr Opin Biotechnol 14:659–664
Parhi R, Suresh P (2012) Preparation and characterisation of solid lipid nanoparticles-a review. Curr Drug Discov Technol 9:2–16
Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8:147–166
Perkins AC, Frier M (2004) Radionuclide imaging in drug development. Curr Pharm Des 10:2907–2921
Semete B, Booysen L, Lemmer Y, Kalombo L, Venter J, Katata L, Verschoor J, Swai H (2010) In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems, Nanomedicine 6:662–671
Smola M, Vandamme T, Sokolowski A (2008) Nanocarriers aspulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomed 3(1):1–19
Venter L, Jansen D, Wagener J, Fourie PB, Zeevaart JR (2012) Characterization of radiolabelled dry powder leucine, a constituent of inhalable capreomycin. Drug Deliv Lett 2:155–161
Vergoni AV, Tosi G, Tacchi R, VAndelli MA, Bertolini A, Costantino L (2009) Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomed Nanotechnol Biol Med 5:369–377
Yeong CH, Abdullah BJJ, Ng KH, Chung LY, Goh KL, Sarji SA, Perkins AC (2011a) Neutron activated 153Sm-ion-exchange resin as a tracer for gastrointestinal Scintigraphy. Nucl Med Commun 32(12):1256–1260
Yeong CH, Blackshaw PE, Ng KH, Abdullah BJJ, Blaauw M, Dansereau RJ, Perkins AC (2011b) Reproductivity of neutron activated Sm-153 oral dose formulations intended for human administration. Appl Radiat Isot 69(9):1181–1184
Yeong CH, Abdullah BJJ, Ng KH, Chung LY, Goh KL, Sarji SA, Perkins AC (2012) Production and first use of 153SmCl3-ion exchange resin capsule formulation for assessing gastrointestinal motility. Appl Radiat Isot 70:450–455
Acknowledgments
The authors would like to thank the Nuclear Technologies in Medicine and the Biosciences Initiative (NTeMBI), a national technology platform developed and managed by the South African Nuclear Energy Corporation (Necsa) and funded by the Department of Science and Technology. A special thanks to Cindy Els and Delene van Wyk at the Steve Biko Academic Hospital for assisting with the scintigraphic imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandiwana, V., Kalombo, L., Venter, K. et al. Samarium oxide as a radiotracer to evaluate the in vivo biodistribution of PLGA nanoparticles. J Nanopart Res 17, 375 (2015). https://doi.org/10.1007/s11051-015-3182-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3182-3