Abstract
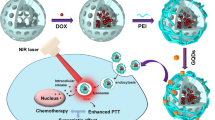
Graphene oxide (GO) with strong optical absorption in the near-infrared (NIR) region has shown great potential both in photothermal therapy and drug delivery. In this work, hyaluronic acid (HA)-functionalized GO (HA-GO) was successfully synthesized and controlled loading of mitoxantrone (MIT) onto HA-GO via π–π stacking interaction was investigated. The results revealed that drug-loaded nanosheets with high loading efficiency of 45 wt% exhibited pH-sensitive responses to tumor environment. Owing to the receptor-mediated endocytosis, cellular uptake analysis of HA-GO showed enhanced internalization. In vivo optical imaging test demonstrated that HA-GO nanosheets could enhance the targeting ability and residence time in tumor site. Moreover, the anti-tumor activity of free MIT, MIT/GO, and MIT/HA-GO in combination with NIR laser was investigated using human MCF-7 cells. In vitro cytotoxicity study revealed that HA-GO could stand as a biocompatible nanocarrier and MIT/HA-GO demonstrated remarkably higher toxicity than free MIT and MIT/GO, with IC50 of 0.79 µg ml−1. Tumor cell-killing potency was enhanced when MIT/HA-GO were combined with NIR irradiation, and the IC50 of MIT/HA-GO plus laser irradiation was 0.38 µg ml−1. In vivo, MIT/HA-GO plus NIR laser irradiation with the tumor growth inhibition of 93.52 % displayed greater anti-tumor effect compared with free MIT and MIT/GO with or without laser irradiation. Therefore, the MIT/HA-GO nanosheets may potentially be useful for further development of synergistic cancer therapy.











Similar content being viewed by others
References
Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, Naito Y, Yoshikawa T (2009) Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperth 25:210–219
An G, Morris ME (2010) HPLC analysis of mitoxantrone in mouse plasma and tissues: application in a pharmacokinetic study. J Pharm Biomed Anal 51:750–753
Boca SC, Potara M, Gabudean A-M, Juhem A, Baldeck PL, Astilean S (2011) Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett 311:131–140
Cho YW, Park SA, Han TH, Son DH, Park JS, Oh SJ, Moon DH, Cho KJ, Ahn CH, Byun Y et al (2007) In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials 28:1236–1247
Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY (2010) Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 31:106–114
Entwistle J, Hall CL, Turley EA (1996) HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem 61:569–577
Gillies ER, Frechet JM (2005) pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug Chem 16:361–368
He X, Wolkers WF, Crowe JH, Swanlund DJ, Bischof JC (2004) In situ thermal denaturation of proteins in dunning AT-1 prostate cancer cells: implication for hyperthermic cell injury. Ann Biomed Eng 32:1384–1398
He M, Zhao Z, Yin L, Tang C, Yin C (2009) Hyaluronic acid coated poly(butyl cyanoacrylate) nanoparticles as anticancer drug carriers. Int J Pharm 373:165–173
Hou L, Yao J, Zhou J, Zhang Q (2012) Pharmacokinetics of a paclitaxel-loaded low molecular weight heparin-all-trans-retinoid acid conjugate ternary nanoparticulate drug delivery system. Biomaterials 33:5431–5440
Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, Zhou X, Guo S, Cui D (2011) Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics 1:240–250
Liang C, Li H, Wang L, Chen X, Zhao W (2010) Investigation of the cytotoxicity of carbon nanotubes using hydroxyapatite as a nano-matrix towards mouse fibroblast cells. Mater Chem Phys 124:21–24
Liu J, Ohta S, Sonoda A, Yamada M, Yamamoto M, Nitta N, Murata K, Tabata Y (2007) Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J Control Release 117:104–110
Liu H, Chen D, Li L, Liu T, Tan L, Wu X, Tang F (2011a) Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed Engl 50:891–895
Liu X, Tao H, Yang K, Zhang S, Lee S-T, Liu Z (2011b) Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 32:144–151
Liu H, Liu T, Wu X, Li L, Tan L, Chen D, Tang F (2012) Targeting gold nanoshells on silica nanorattles: a drug cocktail to fight breast tumors via a single irradiation with near-infrared laser light. Adv Mater 24:755–761
Melancon MP, Elliott AM, Shetty A, Huang Q, Stafford RJ, Li C (2011a) Near-infrared light modulated photothermal effect increases vascular perfusion and enhances polymeric drug delivery. J Control Release 156:265–272
Melancon MP, Lu W, Zhong M, Zhou M, Liang G, Elliott AM, Hazle JD, Myers JN, Li C, Stafford RJ (2011b) Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials 32:7600–7608
Meng L, Zhang X, Lu Q, Fei Z, Dyson PJ (2012) Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials 33:1689–1698
Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW (2013) Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B 120:156–162
Robinson JT, Tabakman SM, Liang Y, Wang H, Casalongue HS, Vinh D, Dai H (2011) Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc 133:6825–6831
Shan C, Yang H, Han D, Zhang Q, Ivaska A, Niu L (2009) Water-soluble graphene covalently functionalized by biocompatible poly-l-lysine. Langmuir 25:12030–12033
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 1:203–212
Tian B, Wang C, Zhang S, Feng L, Liu Z (2011) Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 5:7000–7009
Upadhyay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, Le Meins JF, Farooque A, Chandraiah G, Jain AK et al (2010) The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 31:2882–2892
Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y (2008) High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C 112:17554–17558
Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z (2010) Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett 10:3318–3323
You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, Liang D, Wei W, Sood AK, Li C (2012) Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J Control Release 158:319–328
Zhang L, Xia J, Zhao Q, Liu L, Zhang Z (2010a) Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6:537–544
Zhang Y, Ali SF, Dervishi E, Xu Y, Li Z, Casciano D, Biris AS (2010b) Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 4:3181–3186
Zhang W, Guo Z, Huang D, Liu Z, Guo X, Zhong H (2011) Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 32:8555–8561
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (81202485 and 81273451).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lin Hou and Qianhua Feng have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, L., Feng, Q., Wang, Y. et al. Multifunctional nanosheets based on hyaluronic acid modified graphene oxide for tumor-targeting chemo-photothermal therapy. J Nanopart Res 17, 162 (2015). https://doi.org/10.1007/s11051-015-2966-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2966-9