Abstract
Background
Cryptococcosis and pneumocystosis are opportunistic infections which are more common in immunosuppressed individuals. Herein, we report a rare case of coinfection of pulmonary cryptococcosis (PC) and Pneumocystis jirovecii pneumonia (PJP) in a patient without a previous predisposing illness.
Case presentation A 76-year-old man was admitted to our hospital with complaints of cough, expectoration, shortness of breath, and fever. A chest computed tomography (CT) showed multiple nodules with diffuse ground-glass opacities (GGOs) in both lungs. The patient was diagnosed as extrinsic allergic alveolitis (Pigeon breeder’s lung). After treatment with corticosteroids, the patient improved with significant absorption of GGOs on chest CT. However, pulmonary nodules gradually enlarged and such lesions could not be explained by EAA. Based on the positivity of serum cryptococcal antigen and pathological examination of lung nodule which confirmed the presence of Cryptococcus spores, PC was diagnosed later and fluconazole was administered. However, repeated chest CT performed about 2 months after antifungal treatment showed significantly increased GGOs in both lungs. The pathological examination of new lung lesions revealed the presence of P. jirovecii. The patient was finally diagnosed having coinfection of PC and PJP and sulfamethoxazole was further prescribed. Thereafter, the patient improved again with significant absorption of GGOs as noted on chest CT.
Conclusions
Concomitant PC and PJP is very rare, especially in a patient without a previous predisposing illness. Additionally, when pulmonary lesions cannot be completely explained by one kind of infectious disease, the possibility of mixed infection should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A 76-year-old man with cough, expectoration, shortness of breath and fever was admitted to our hospital. The patient had no history of any underlying disease. He had been fond of raising pigeons for years. A chest computed tomography (CT) showed multiple nodules with diffuse ground-glass opacities (GGOs) in both lungs (Fig. 1). A decrease in peripheral blood total T lymphocytes (CD3+) and their subtypes (CD4+, CD8+) was noted. CT-guided percutaneous needle biopsy (PNB) of nodule in the left lower lung (Fig. 1, red arrow) was performed, and the pathological examination revealed chronic inflammatory changes with large areas of necrosis. Various special stains were all negative, leading to an indefinite diagnosis. Althouth the patient received anti-infective therapy with moxifloxacin and ganciciovir, his condition continuously deteriorated. Since the patient refused tracheoscopy, bronchoalveolar lavage fluid (BALF) and transbronchial lung biopsy (TBLB) specimens were not available for further diagnosis at that time. Based on diffuse GGOs on chest imaging, a history of pigeon breeding, and the failure of conventional anti-infective therapy, the patient was clinically diagnosed as extrinsic allergic alveolitis (EAA, Pigeon breeder's lung). The empirical anti-inflammatory treatment with methylprednisolone (glucocorticoid) 40 mg once daily was initiated. Two weeks after therapy, repeated chest CT showed significant absorption of GGOs in both lungs. Thereafter, glucocorticoid was discontinued.
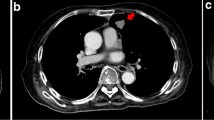
Since pulmonary nodules gradually enlarged and such lesions could not be explained by EAA, an additional detection of serum cryptococcal antigen was performed and the result was positive. Meanwhile, PNB of enlarging nodule in the right upper lung was performed (Fig. 2a, red arrow), and the pathological examination revealed the presence of Cryptococcus spores (Fig. 2b). Lumbar puncture was performed and the results of cerebrospinal fluid testing were normal. The patient was diagnosed as pulmonary cryptococcosis (PC) with multiple nodules and fluconazole 400 mg once daily was initiated. After treatment, chest CT images showed increase in size of nodules in bilateral lung. However, 2 months after antifungal treatment, the patient aggravated again. Repeated chest CT showed new GGOs significantly increased in both lungs (Fig. 3). TBLB was performed in the right upper lung where GGOs significantly progressed (Fig. 3, red arrow). Routine culture of BALF was negative; however, direct Periodic acid-Schiff stain and Gomori's methenamine silver stain on biopsy samples were positive for Pneumocystis jirovecii (Fig. 4). The patient was considered to have coinfection of PC (nodular lesions) and P. jirovecii pneumonia (PJP, GGOs lesions). He was treated with additional compound sulfamethoxazole. Dynamic monitoring chest CT images showed that the lesions including nodules and GGOs continuously absorbed. The patient remained well without signs of recurrence 5 months after onset. Unfortunately, due to the outbreak of coronavirus disease 2019 (COVID-19) pandemic at the end of 2019, the patient was lost to follow-up at last.
Concomitant PC and PJP is very rare, and mainly reported in HIV-infected patients and other immunosuppressed hosts. Surprisingly, this patient did not have definite immunosuppressed underlying diseases. It was considered that PC (nodular lesions) combined with EAA (first appearance of GGOs lesions on admission, Fig. 1) which was induced by allergens from pigeons appeared at first; and secondary PJP (second appearance of GGOs lesions at the late course, Fig. 3) appeared 2 months after anti-cryptococcus treatment. The long-term history of pigeon breeding which might have been associated with PC since pigeon dung has been documented as an important source of infection with Cryptococcus. PJP was probably secondary to corticosteroid therapy in the patient with prealable pneumonia and low lymphocytes count. However, two particular infections were detected in this patient without known predisposing illness, it was speculated that the patient may have an undetected potential immunodeficiency disease. Due to the limitations of our hospital in undertaking detailed laboratory investigations and the patient's financial constraints, the further screening of potential pre-existing systemic immunodeficiency diseases could not be performed. Furthermore, as seen in our case, when pulmonary lesions cannot be completely explained by one kind of infectious disease, the possibility of mixed infection should be considered.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Weng, H., Lan, C. et al. Pulmonary Co-infection with Cryptococcus Species and Pneumocystis jirovecii in an Old Patient Without a Previous Predisposing Illness. Mycopathologia 187, 613–616 (2022). https://doi.org/10.1007/s11046-022-00651-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00651-8