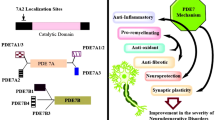
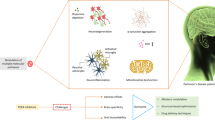
Abstract
Phosphodiesterases (PDEs) have become a promising therapeutic target for various disorders. PDEs are a vast and diversified family of enzymes that degrade cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), which have several biochemical and physiological functions. Phosphodiesterase 4 (PDE4) is the most abundant PDE in the central nervous system (CNS) and is extensively expressed in the mammalian brain, where it catalyzes the hydrolysis of intracellular cAMP. An alteration in the balance of PDE4 and cAMP results in the dysregulation of different biological mechanisms involved in neurodegenerative diseases. By inhibiting PDE4 with drugs, the levels of cAMP inside the cells could be stabilized, which may improve the symptoms of mental and neurological disorders such as memory loss, depression, and Parkinson’s disease (PD). Though numerous studies have shown that phosphodiesterase 4 inhibitors (PDE4Is) are beneficial in PD, there are presently no approved PDE4I drugs for PD. This review presents an overview of PDE4Is and their effects on PD, their possible underlying mechanism in the restoration/protection of dopaminergic cell death, which holds promise for developing PDE4Is as a treatment strategy for PD. Methods on how these drugs could be effectively delivered to develop as a promising treatment for PD have been suggested.




Similar content being viewed by others
Data availability
No Data associated in the manuscript.
References
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42. https://doi.org/10.1111/ene.14108
Haobam R, Sindhu KM, Chandra G, Mohanakumar KP (2005) Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav Brain Res 163(2):159–167. https://doi.org/10.1016/j.bbr.2005.04.011
Khan ST, Ahmed S, Gul S, Khan A, Al-Harrasi A (2021) Search for safer and potent natural inhibitors of Parkinson’s disease. Neurochem Int 149:105135. https://doi.org/10.1016/j.neuint.2021.105135
Naoi M, Maruyama W (1999) Cell death of dopamine neurons in aging and Parkinson’s disease. Mech Ageing Dev 111(2–3):175–188. https://doi.org/10.1016/S0047-6374(99)00064-0
Petese A, Cesaroni V, Cerri S, Blandini F (2022) Are Lysosomes Potential Therapeutic Targets for Parkinson’s Disease? CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 21(8), pp.642–655. https://doi.org/10.2174/1871527320666210809123630
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139:318–324. https://doi.org/10.1111/jnc.13691
Vijiaratnam N, Foltynie T (2020) Therapeutic strategies to treat or prevent off episodes in adults with Parkinson’s disease. Drugs 80:775–796. https://doi.org/10.1007/s40265-020-01310-2
Kostrzewa RM, Kostrzewa JP, Brus R (2002) Neuroprotective and neurotoxic roles of levodopa (L-DOPA) in neurodegenerative disorders relating to Parkinson’s disease. Amino Acids 23:57–63. https://doi.org/10.1007/s00726-001-0110-x
Chen JY, Zhu Q, Cai CZ, Luo HB, Lu JH (2022) α-mangostin derivative 4e as a PDE4 inhibitor promote proteasomal degradation of alpha-synuclein in Parkinson’s disease models through PKA activation. Phytomedicine 101:154125. https://doi.org/10.1016/j.phymed.2022.154125
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37(3):510–518. https://doi.org/10.1016/j.nbd.2009.11.004
Cao M, Wu Y, Ashrafi G, McCartney AJ, Wheeler H, Bushong EA, Boassa D, Ellisman MH, Ryan TA, De Camilli P (2017) Parkinson Sac domain mutation in synaptojanin 1 impairs clathrin uncoating at synapses and triggers dystrophic changes in dopaminergic axons. Neuron 93(4):882–896. https://doi.org/10.1016/j.neuron.2017.01.019
Leszek J, Barreto E, Gasiorowski G, Koutsouraki K, E. and, Aliev G (2016) Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol Disorders-Drug Targets (Formerly Curr Drug Targets-CNS Neurol Disorders) 15(3):329–336. https://doi.org/10.2174/1871527315666160202125914
Zhong J, Xie J, Xiao J, Li D, Xu B, Wang X, Wen H, Zhou Z, Cheng Y, Xu J, Wang H (2019) Inhibition of PDE4 by FCPR16 induces AMPK-dependent autophagy and confers neuroprotection in SH-SY5Y cells and neurons exposed to MPP+-induced oxidative insult. Free Radic Biol Med 135:87–101. https://doi.org/10.1016/j.freeradbiomed.2019.02.027
Nthenge-Ngumbau DN, Mohanakumar KP (2018) Can cyclic nucleotide phosphodiesterase inhibitors be drugs for Parkinson’s disease? Mol Neurobiol 55:822–834. https://doi.org/10.1007/s12035-016-0355-8
Heckman PRA, Blokland A, Bollen EPP, Prickaerts J (2018) Phosphodiesterase inhibition and modulation of corticostriatal and hippocampal circuits: clinical overview and translational considerations. Neurosci Biobehavioral Reviews 87:233–254. https://doi.org/10.1016/j.neubiorev.2018.02.007
Padda IS, Tripp PJ (2021) Phosphodiesterase Inhibitors (Treasure Island, FL: StatPearls), 1–6
Crocetti L, Floresta G, Cilibrizzi A, Giovannoni MP (2022) An overview of PDE4 inhibitors in clinical trials: 2010 to early 2022. Molecules 27(15):4964. https://doi.org/10.3390/molecules27154964
Wei X, Yu G, Shen H, Luo Y, Shang T, Shen R, Xi M, Sun H (2023) Targeting phosphodiesterase 4 as a therapeutic strategy for cognitive improvement. Bioorg Chem 130:106278. https://doi.org/10.1016/j.bioorg.2022.106278
Zhu J, Mix E, Winblad B (2001) The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev 7(4):387–398. https://doi.org/10.1111/j.1527-3458.2001.tb00206.x
Lugnier C (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109(3):366–398. https://doi.org/10.1016/j.pharmthera.2005.07.003
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58(3):488–520. https://doi.org/10.1124/pr.58.3.5
Zhang HT (2009) Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Design 15(14):1688–1698. https://doi.org/10.2174/138161209788168092
Houslay MD, Schafer P, Zhang KY (2005) Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discovery Today 10(22):1503–1519. https://doi.org/10.1016/S1359-6446(05)03622-6
Houslay MD (2010) Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci 35(2):91–100. https://doi.org/10.1016/j.tibs.2009.09.007
Nishi A, Snyder GL (2010) Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J Pharmacol Sci 114(1):6–16. https://doi.org/10.1254/jphs.10R01FM
Nobuyuki Y, Akiko H, Jun B, Aiko S (1997) Rolipram, a phosphodiesterase-4-selective inhibitor, promotes the survival of cultured rat dopaminergic neurons. Jpn J Pharmacol 75(2):155–159. https://doi.org/10.1016/S0021-5198(19)31327-7
Heckman PR, Van Duinen MA, Bollen EP, Nishi A, Wennogle LP, Blokland A, Prickaerts J (2016) Phosphodiesterase inhibition and regulation of dopaminergic frontal and striatal functioning: clinical implications. Int J Neuropsychopharmacol 19(10):pyw030. https://doi.org/10.1093/ijnp/pyw030
Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296(5573):1636–1639. https://doi.org/10.1126/science.1071550
Giorgi M, D’Angelo V, Esposito Z, Nuccetelli V, Sorge R, Martorana A, Stefani A, Bernardi G, Sancesario G (2008) Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: new aspects in the pathogenetic mechanisms. Eur J Neurosci 28(5):941–950. https://doi.org/10.1111/j.1460-9568.2008.06387.x
Keravis T, Lugnier C (2010) Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr Pharm Design 16(9):1114–1125. https://doi.org/10.2174/138161210790963760
Wang H, Gaur U, Xiao J, Xu B, Xu J, Zheng W (2018) Targeting phosphodiesterase 4 as a potential therapeutic strategy for enhancing neuroplasticity following ischemic stroke. Int J Biol Sci 14(12):1745. https://doi.org/10.7150/ijbs.26230
Bhat A, Ray B, Mahalakshmi AM, Tuladhar S, Nandakumar DN, Srinivasan M, Essa MM, Chidambaram SB, Guillemin GJ, Sakharkar MK (2020) Phosphodiesterase-4 enzyme as a therapeutic target in neurological disorders. Pharmacol Res 160:105078. https://doi.org/10.1016/j.phrs.2020.105078
Alberini CM (2009) Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89(1):121–145. https://doi.org/10.1152/physrev.00017.2008
Singh AK, Kashyap MP, Tripathi VK, Singh S, Garg G, Rizvi SI (2017) Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol Neurobiol 54:5815–5828. https://doi.org/10.1007/s12035-016-0129-3
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science 286(5443):1358–1362. https://doi.org/10.1126/science.286.5443.1358
Richter W, Menniti FS, Zhang HT, Conti M (2013) PDE4 as a target for cognition enhancement. Expert Opin Ther Targets 17(9):1011–1027. https://doi.org/10.1517/14728222.2013.818656
Liu Z, Liu M, Cao Z, Qiu P, Song G (2022) Phosphodiesterase-4 inhibitors: a review of current developments (2013–2021). Expert Opin Ther Pat 32(3):261–278. https://doi.org/10.1080/13543776.2022.2026328
Titus DJ, Wilson NM, Alcazar O, Calixte DA, Dietrich WD, Gurney ME, Atkins CM (2018) A negative allosteric modulator of PDE4D enhances learning after traumatic brain injury. Neurobiol Learn Mem 148:38–49. https://doi.org/10.1016/j.nlm.2017.12.008
Gurney ME, Burgin AB, Magnusson OT, Stewart LJ (2011) Small molecule allosteric modulators of phosphodiesterase 4. Phosphodiesterases as Drug Targets 167–192. https://doi.org/10.1007/978-3-642-17969-3_7
Xia C, He JP, Feng KW, Liu L, Zheng L, Wang HT, Xu JP, Zhou ZZ (2022) Discovery of novel 3-Amino-4-alkoxyphenylketones as PDE4 inhibitors with improved oral bioavailability and safety against spatial memory impairments. ACS Chem Neurosci 13(3):390–405. https://doi.org/10.1021/acschemneuro.1c00762
Correia AC, Monteiro AR, Silva R, Moreira JN, Lobo JS, Silva AC (2022) Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv Drug Deliv Rev 114485. https://doi.org/10.1016/j.addr.2022.114485
Khan AR, Yang X, Fu M, Zhai G (2018) Recent progress of drug nanoformulations targeting to brain. J Controlled Release 291:37–64. https://doi.org/10.1016/j.jconrel.2018.10.004
Sola P, Krishnamurthy P, Chintamaneni PK, Pindiprolu SKS, Kumari M (2020) Novel drug delivery systems of β2 adrenoreceptor agonists to suppress SNCA gene expression and mitochondrial oxidative stress in Parkinson’s disease management. Expert Opin Drug Deliv 17(8):1119–1132. https://doi.org/10.1080/17425247.2020.1779218
Bhat A, Tan V, Heng B, Lovejoy DB, Sakharkar MK, Essa MM, Chidambaram SB, Guillemin GJ (2020) Roflumilast, a cAMP-specific phosphodiesterase-4 inhibitor, reduces oxidative stress and improves synapse functions in human cortical neurons exposed to the excitotoxin quinolinic acid. ACS Chem Neurosci 11(24):4405–4415. https://doi.org/10.1021/acschemneuro.0c00636
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. https://doi.org/10.1146/annurev-neuro-061010-113641
Paramo B, Wyatt S, Davies AM (2019) Neuregulin-4 is required for the growth and elaboration of striatal medium spiny neuron dendrites. J Neuropathology Experimental Neurol 78(8):725–734. https://doi.org/10.1093/jnen/nlz046
Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296. https://doi.org/10.1146/annurev.pharmtox.44.101802.121415
Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116(1):1–9. https://doi.org/10.1111/j.1471-4159.2010.07080.x
Pearse DD, Hughes ZA (2016) PDE4B as microglia target to reduce neuroinflammation. Glia 64(10):1698–1709. https://doi.org/10.1002/glia.22986
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. https://doi.org/10.1016/j.biopha.2015.07.025
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxidative Medicine and Cellular Longevity, 2012. https://doi.org/10.1155/2012/428010
Jenner P (2003) Oxidative stress in Parkinson’s disease. Annals Neurology: Official J Am Neurol Association Child Neurol Soc 53(S3):S26–S38. https://doi.org/10.1002/ana.10483
Xu B, Wang T, Xiao J, Dong W, Wen HZ, Wang X, Qin Y, Cai N, Zhou Z, Xu J, Wang H (2019) FCPR03, a novel phosphodiesterase 4 inhibitor, alleviates cerebral ischemia/reperfusion injury through activation of the AKT/GSK3β/β-catenin signaling pathway. Biochem Pharmacol 163:234–249. https://doi.org/10.1016/j.bcp.2019.02.023
Zhong J, Li M, Xu J, Dong W, Qin Y, Qiu S, Li X, Wang H (2022) Roflupram attenuates α-synuclein-induced cytotoxicity and promotes the mitochondrial translocation of Parkin in SH-SY5Y cells overexpressing A53T mutant α-synuclein. Toxicol Appl Pharmcol 436:115859. https://doi.org/10.1016/j.taap.2021.115859
Zhong J, Dong W, Qin Y, Xie J, Xiao J, Xu J, Wang H (2020) Roflupram exerts neuroprotection via activation of CREB/PGC-1α signalling in experimental models of Parkinson’s disease. Br J Pharmacol 177(10):2333–2350. https://doi.org/10.1111/bph.14983
Zhong J, Yu H, Huang C, Zhong Q, Chen Y, Xie J, Zhou Z, Xu J, Wang H (2018) Inhibition of phosphodiesterase 4 by FCPR16 protects SH-SY5Y cells against MPP+-induced decline of mitochondrial membrane potential and oxidative stress. Redox Biol 16:47–58. https://doi.org/10.1016/j.redox.2018.02.008
Casacchia M, Meco G, Castellana F, Bedini L, Cusimano G, Agnoli A (1983) Therapeutic use of a selective cAMP phosphodiesterase inhibitor (Rolipram) in Parkinson’s disease. Pharmacol Res Commun 15(3):329–334. https://doi.org/10.1016/S0031-6989(83)80017-4
Wilson H, Pagano G, Niccolini F, Muhlert N, Mehta MA, Searle G, Gunn RN, Rabiner EA, Foltynie T, Politis M (2020) The role of phosphodiesterase 4 in excessive daytime sleepiness in Parkinson’s disease. Parkinsonism Relat Disord 77:163–169. https://doi.org/10.1016/j.parkreldis.2019.02.027
Liu H, Wu H, Zhu N, Xu Z, Wang Y, Qu Y, Wang J (2020) Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl‐4‐phenyl‐1, 2, 3, 6‐tetrahydropyridine (MPTP)‐induced Parkinson’s disease in mice. J Neurochem 152(3):397–415. https://doi.org/10.1111/jnc.14857
Yang L, Matthews RT, Schulz JB, Klockgether T, Liao AW, Martinou JC, Penney JB, Hyman BT, Beal MF (1998) 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci 18(20):8145–8152. https://doi.org/10.1523/JNEUROSCI.18-20-08145.1998
Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ (2008) Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci 105(35):12815–12819. https://doi.org/10.1073/pnas.0707715105
Timmons S, Coakley MF, Moloney AM, O’Neill C (2009) Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett 467(1):30–35. https://doi.org/10.1016/j.neulet.2009.09.055
Huang N, Zhang Y, Chen M, Jin H, Nie J, Luo Y, Zhou S, Shi J, Jin F (2019) Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp Gerontol 124:110653. https://doi.org/10.1016/j.exger.2019.110653
Lu XL, Zhao CH, Yao XL, Zhang H (2017) Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed Pharmacother 85:658–671. https://doi.org/10.1016/j.biopha.2016.11.077
Xu B, Qin Y, Li D, Cai N, Wu J, Jiang L, Jie L, Zhou Z, Xu J, Wang H (2020) Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol 28:101342. https://doi.org/10.1016/j.redox.2019.101342
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14(7):463–477. https://doi.org/10.1038/nri3705
Doorn KJ, Moors T, Drukarch B, van de Berg WD, Lucassen PJ, van Dam AM (2014) Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun 2(1):1–17. https://doi.org/10.1186/s40478-014-0090-1
Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, Carta AR (2014) Dynamic changes in pro-and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis 71:280–291. https://doi.org/10.1016/j.nbd.2014.08.011
Ghosh M, Garcia-Castillo D, Aguirre V, Golshani R, Atkins CM, Bramlett HM, Dietrich WD, Pearse DD (2012) Proinflammatory cytokine regulation of cyclic AMP‐phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia 60(12):1839–1859. https://doi.org/10.1002/glia.22401
Morales-Garcia JA, Redondo M, Alonso-Gil S, Gil C, Perez C, Martinez A, Santos A, Perez-Castillo A (2011) Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease. PLoS ONE 6(2):e17240. https://doi.org/10.1371/journal.pone.0017240
Kambayashi T, Jacob CO, Zhou D, Mazurek N, Fong M, Strassmann G (1995) Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J Immunol (Baltimore Md : 1950) 155(10):4909–4916. https://doi.org/10.4049/jimmunol.155.10.4909
Jin SLC, Conti M (2002) Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-α responses. Proceedings of the National Academy of Sciences, 99(11), pp.7628–7633. https://doi.org/10.1073/pnas.122041599
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC (2014) Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discovery 13(4):290–314. https://doi.org/10.1038/nrd4228
Zou ZQ, Chen JJ, Feng HF, Cheng YF, Wang HT, Zhou ZZ, Guo HB, Zheng W, Xu JP (2017) Novel phosphodiesterase 4 inhibitor FCPR03 alleviates lipopolysaccharide-induced neuroinflammation by regulation of the cAMP/PKA/CREB signaling pathway and NF-κB inhibition. J Pharmacol Exp Ther 362(1):67–77. https://doi.org/10.1124/jpet.116.239608
Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y (2001) Increase in peripheral CD4 bright + CD8 dull + T cells in Parkinson disease. Arch Neurol 58(10):1580–1583. https://doi.org/10.1001/archneur.58.10.1580
Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M (2009) Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23(1):55–63. https://doi.org/10.1016/j.bbi.2008.07.003
You T, Cheng Y, Zhong J, Bi B, Zeng B, Zheng W, Wang H, Xu J (2017) Roflupram, a phosphodiesterase 4 inhibitior, suppresses inflammasome activation through autophagy in microglial cells. ACS Chem Neurosci 8(11):2381–2392. https://doi.org/10.1021/acschemneuro.7b00065
Schwenkgrub J, Zaremba M, Joniec-Maciejak I, Cudna A, Mirowska-Guzel D, Kurkowska-Jastrzębska I (2017) The phosphodiesterase inhibitor, ibudilast, attenuates neuroinflammation in the MPTP model of Parkinson’s disease. PLoS ONE 12(7):e0182019. https://doi.org/10.1371/journal.pone.0182019
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164(4):1357–1391. https://doi.org/10.1111/j.1476-5381.2011.01426.x
Samidurai A, Xi L, Das A, Iness AN, Vigneshwar NG, Li PL, Singla DK, Muniyan S, Batra SK, Kukreja RC (2021) Role of phosphodiesterase 1 in the pathophysiology of diseases and potential therapeutic opportunities. Pharmacol Ther 226:107858. https://doi.org/10.1016/j.pharmthera.2021.107858
Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Liu W, Schioth HB (2022) Recent developments of phosphodiesterase inhibitors: clinical trials, emerging indications and novel molecules. Front Pharmacol 13:1057083. https://doi.org/10.3389/fphar.2022.1057083
Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM (2005) Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci CMLS 62:1198–1220. https://doi.org/10.1007/s00018-005-4533-5
Zhang C, Xu Y, Zhang HT, Gurney ME, O’Donnell JM (2017) Comparison of the pharmacological profiles of selective PDE4B and PDE4D inhibitors in the central nervous system. Sci Rep 7(1):40115. https://doi.org/10.1038/srep40115
Xu RX, Hassell AM, Vanderwall D, Lambert MH, Holmes WD, Luther MA, Rocque WJ, Milburn MV, Zhao Y, Ke H, Nolte RT (2000) Atomic structure of PDE4: insights into phosphodiesterase mechanism and specificity. Science 288(5472):1822–1825. https://doi.org/10.1126/science.288.5472.1822
Zhuo Y, Guo H, Cheng Y, Wang C, Wang C, Wu J, Zou Z, Gan D, Li Y, Xu J (2016) Inhibition of phosphodiesterase-4 reverses the cognitive dysfunction and oxidative stress induced by Aβ25–35 in rats. Metab Brain Dis 31:779–791. https://doi.org/10.1007/s11011-016-9814-1
Bruno O, Fedele E, Prickaerts J, Parker LA, Canepa E, Brullo C, Cavallero A, Gardella E, Balbi A, Domenicotti C, Bollen E (2011) GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non‐emetic doses. Br J Pharmacol 164(8):2054–2063. https://doi.org/10.1111/j.1476-5381.2011.01524.x
Gilleen J, Farah Y, Davison C, Kerins S, Valdearenas L, Uz T, Lahu G, Tsai M, Ogrinc F, Reichenberg A, Williams SC (2021) An experimental medicine study of the phosphodiesterase-4 inhibitor, roflumilast, on working memory-related brain activity and episodic memory in schizophrenia patients. Psychopharmacology 238:1279–1289. https://doi.org/10.1007/s00213-018-5134-y
Kraft P, Schwarz T, Göb E, Heydenreich N, Brede M, Meuth SG, Kleinschnitz C (2013) The phosphodiesterase-4 inhibitor rolipram protects from ischemic stroke in mice by reducing blood–brain-barrier damage, inflammation and thrombosis. Exp Neurol 247:80–90. https://doi.org/10.1016/j.expneurol.2013.03.026
Jabaris SSL, Sumathy H, Girish R, Narayanan S, Sugumar M, Babu CS, Thanikachalam S, Thanikachalam M (2015) Phosphodiesterase-4 inhibitors ameliorates cognitive deficits in deoxycorticosterone acetate induced hypertensive rats via cAMP/CREB signaling system. Brain Res 1622:279–291. https://doi.org/10.1016/j.brainres.2015.07.003
González-García C, Bravo B, Ballester A, Gómez‐Pérez R, Eguiluz C, Redondo M, Martínez A, Gil C, Ballester S (2013) Comparative assessment of PDE 4 and 7 inhibitors as therapeutic agents in experimental autoimmune encephalomyelitis. Br J Pharmacol 170(3):602–613. https://doi.org/10.1111/bph.12308
Li YF, Huang Y, Amsdell SL, Xiao L, O’donnell JM, Zhang HT (2009) Antidepressant-and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34(11):2404–2419. https://doi.org/10.1038/npp.2009.66
Yu Y, Wang J, Huang X (2021) The anti-depressant effects of a novel PDE4 inhibitor derived from resveratrol. Pharm Biol 59(1):416–421. https://doi.org/10.1080/13880209.2021.1907422
Wilson NM, Titus DJ, Oliva AA Jr, Furones C, Atkins CM (2016) Traumatic brain injury upregulates phosphodiesterase expression in the hippocampus. Front Syst Neurosci 10:5. https://doi.org/10.3389/fnsys.2016.00005
DeMarch Z, Giampà C, Patassini S, Martorana A, Bernardi G, Fusco FR (2007) Beneficial effects of rolipram in a quinolinic acid model of striatal excitotoxicity. Neurobiol Dis 25(2):266–273. https://doi.org/10.1016/j.nbd.2006.09.006
DeMarch Z, Giampà C, Patassini S, Bernardi G, Fusco FR (2008) Beneficial effects of rolipram in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis 30(3):375–387. https://doi.org/10.1016/j.nbd.2008.02.010
Huang H, Hong Q, Tan HL, Xiao CR, Gao Y (2016) Ferulic acid prevents LPS-induced up-regulation of PDE4B and stimulates the cAMP/CREB signaling pathway in PC12 cells. Acta Pharmacol Sin 37(12):1543–1554. https://doi.org/10.1038/aps.2016.88
Dong WL, Zhong JH, Chen YQ, Xie JF, Qin YY, Xu JP, Cai NB, Li MF, Liu L, Wang HT (2021) Roflupram protects against rotenone-induced neurotoxicity and facilitates α-synuclein degradation in Parkinson’s disease models. Acta Pharmacol Sin 42(12):1991–2003. https://doi.org/10.1038/s41401-021-00768-4
Kinoshita KI, Muroi Y, Unno T, Ishii T (2017) Rolipram improves facilitation of contextual fear extinction in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. J Pharmacol Sci 134(1):55–58. https://doi.org/10.1016/j.jphs.2017.04.002
Hulley P, Hartikka J, Abdel’Al S, Engels P, Buerki HR, Wiederhold KH, Müller T, Kelly P, Lowe D, Lübbert H (1995) Inhibitors of type IV phosphodiesterases reduce the toxicity of MPTP in Substantia Nigra neurons in vivo. Eur J Neurosci 7(12):2431–2440. https://doi.org/10.1111/j.1460-9568.1995.tb01041.x
Yang L, Calingasan NY, Lorenzo BJ, Beal MF (2008) Attenuation of MPTP neurotoxicity by rolipram, a specific inhibitor of phosphodiesterase IV. Exp Neurol 211(1):311–314. https://doi.org/10.1016/j.expneurol.2007.02.010
Sanders O, Rajagopal L (2020) Phosphodiesterase inhibitors for Alzheimer’s disease: a systematic review of clinical trials and epidemiology with a mechanistic rationale. J Alzheimer’s Disease Rep 4(1):185–215. https://doi.org/10.3233/adr-200191
Prickaerts J, Heckman PR, Blokland A (2017) Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin Investig Drugs 26(9):1033–1048. https://doi.org/10.1080/13543784.2017.1364360
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Pooja Devi Nongthombam and Reena Haobam conceptualized the ideas in the manuscript. Reena Haobam and Pooja Devi Nongthombam contributed to the collection and analyses of materials and to the preparation of the manuscript. Both authors have gone through and agreed to the submitted version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nongthombam, P.D., Haobam, R. Targeting phosphodiesterase 4 as a potential therapy for Parkinson’s disease: a review. Mol Biol Rep 51, 510 (2024). https://doi.org/10.1007/s11033-024-09484-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09484-8