Abstract
Background
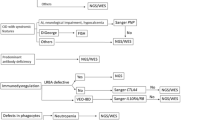
Severe Combined Immunodeficiency (SCID) is an autosomal recessive inborn error of immunity (IEI) characterized by recurrent chest and gastrointestinal (GI) infections and in some cases associated with life-threatening disorders.
Methodology and results
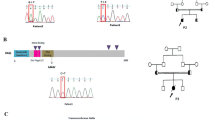
This current study aims to unwind the molecular etiology of SCID and also extended the patients’ phenotype associated with identified particular variants. Herein, we present 06 disease-causing variants identified in 07 SCID-patients in three different SCID related genes. Whole Exome Sequencing (WES) followed by Sanger Sequencing was employed to explore genetic variations. The results included identification of two previously reported heterozygous variants in homozygous form for the first time in RAG1gene [(p.Arg410Gln);(p.Arg737His)], followed by a recurrent variant (p.Trp959*) in RAG1, a novel variant in IL2RG (p.Asp48Lfs*24), a recurrent variant in IL2RG (p.Gly271Glu) and a recurrent variant in DCLRE1C (p.Arg191*) gene.
Conclusion
To conclude, the immune-profiling and WES revealed two novel, two as homozygous state for the first time, and two recurrent disease causing variants contributing valuably to our existing knowledge of SCID.



Similar content being viewed by others
Data availability
Data will be provided upon request.
References
Pieniawska-Śmiech K, Pasternak G, Lewandowicz-Uszyńska A, Jutel M (2022) Diagnostic challenges in patients with inborn errors of immunity with different manifestations of immune dysregulation. J Clin Med 11(14):4220
Yamashita M, Inoue K, Okano T et al (2021) Inborn errors of immunity—recent advances in research on the pathogenesis. Inflamm Regener 41:9
Abolhassani H, Azizi G, Sharifi L, Yazdani R, Mohsenzadegan M, Delavari S, Aghamohammadi A (2020) Global systematic review of primary immunodeficiency registries. Expert Rev Clin Immunol 16(7):717–732
Wadbudhe AM, Meshram RJ, Tidke SC (2023) Severe combined immunodeficiency (SCID) and its new treatment modalities. Cureus. https://doi.org/10.7759/cureus.47759
Kumrah R, Vignesh P, Patra P, Singh A, Anjani G, Saini P, Rawat A (2020) Genetics of severe combined immunodeficiency. Genes Dis 7(1):52–61
Dvorak CC, Haddad E, Heimall J, Dunn E, Cowan MJ, Pai SY, Puck JM (2023) The diagnosis of severe combined immunodeficiency: implementation of the PIDTC 2022 definitions. J Allergy Clin Immunol 151(2):547–555
Zhang Q, Frange P, Blanche S, Casanova JL (2017) Pathogenesis of infections in HIV-infected individuals: insights from primary immunodefciencies. Curr Opin Immunol 48:122–133
Tangye SG, Al-Herz W, Bousfha A, Chatila T, Cunningham-Rundles C, Etzioni A et al (2020) Human inborn errors of immunity: 2019 update on the classifcation from the international union of immunological societies expert committee. J Clin Immunol 40(1):24–64
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164–e164
Kircher M, Witten DM, Jain P, O’roak BJ, Cooper GM, Shendure J (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46:310–315
Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, Tangye SG (2020) Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol 40:66–81
Lee YN, Frugoni F, Dobbs K, Tirosh I, Du L, Ververs FA, Notarangelo LD (2016) Characterization of T and B cell repertoire diversity in patients with RAG deficiency. Sci Immunol 1(6):6109
Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, Schwarz K (2001) V (D) J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 97(1):81–88
Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, Notarangelo LD (2014) A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol 133(4):1099–1108
Kumánovics A, Lee YN, Close DW, Coonrod EM, Ujhazi B, Chen K, Walter JE (2017) Estimated disease incidence of RAG1/2 mutations: a case report and querying the exome aggregation consortium. J Allergy Clin Immunol 139(2):690–692
Villa A, Santagata S, Bozzi F, Giliani S, Frattini A, Imberti L, Spanopoulou E (1998) Partial V (D) J recombination activity leads to omenn syndrome. Cell 93(5):885–896
Schuetz C, Gudowius S, Niehues T, Schulz A, Schwarz K, Debatin KM, Friedrich W (2005) Compound heterozygeous RAG-1 mutations in 2 patients presenting with chronic disfiguring granulomatous skin lesions. Blood 106(11):3898
Inglis AJ, Guna A, Gálvez-Merchán Á, Pal A, Esantsi TK, Keys HR, Voorhees RM (2023) Coupled protein quality control during nonsense-mediated mRNA decay. J Cell Sci 136(10):jcs261216
Puck JM, Pepper AE, Henthorn PS, Candotti F, Isakov J, Whitwam T, Buckley RH (1997) Mutation analysis of IL2RG in human X-linked severe combined immunodeficiency. Blood 89(6):1968–1977
Halacli SO (2022) The effect of mutatio-type on proteo-phenotype and clinico-phenotype in selected primary immunodeficiencies. Immunol Res 70(1):56–66
Niemela JE, Puck JM, Fischer RE, Fleisher TA, Hsu AP (2000) Efficient detection of thirty-seven new IL2RG mutations in human X-linked severe combined immunodeficiency. Clin Immunol 95(1):33–38
Ott N, Faletti L, Heeg M, Andreani V, Grimbacher B (2023) JAKs and STATs from a clinical perspective: loss-of-function mutations, gain-of-function mutations, and their multidimensional consequences. J Clin Immunol 43(6):1326–1359
Gaikwad P, Bargir UA, Shinde S, Kini P, Chaurasia R, Yadav U, Madkaikar M (2023) A Clinical conundrum with diagnostic and therapeutic challenge: a tale of two disorders in one case. J Clin Immunol 43:1891
Volk T, Pannicke U, Reisli I, Bulashevska A, Ritter J, Björkman A, Grimbacher B (2015) DCLRE1C (ARTEMIS) mutations causing phenotypes ranging from atypical severe combined immunodeficiency to mere antibody deficiency. Hum Mol Genet 24(25):7361–7372
Felgentreff K, Lee YN, Frugoni F, Du L, van der Burg M, Giliani S, Notarangelo LD (2015) Functional analysis of naturally occurring DCLRE1C mutations and correlation with the clinical phenotype of ARTEMIS deficiency. J Allergy Clin Immunol 136(1):140–150
Platt CD, Zaman F, Bainter W, Stafstrom K, Almutairi A, Reigle M, Weeks S, Geha RS, Chou J (2021) Efficacy and economics of targeted panel versus whole-exome sequencing in 878 patients with suspected primary immunodeficiency. J Allergy Clin Immunol 147(2):723–726. https://doi.org/10.1016/j.jaci.2020.08.022
Funding
The authors (Hajra Fayyaz and Atteaya Zaman) were supported by IRSIP fellowships from Higher Education Commission (HEC), Islamabad, Pakistan.
Author information
Authors and Affiliations
Contributions
Hajra Fayyaz and Atteaya Zaman collected blood samples, performed experimental work and prepared the manuscript. Sheeba Shabbir, Zara Khalid, Nighat Haider, and Ali Faisal Saleem identified the families and help in diagnosis of disease phenotypes. Wasim Ahmad and Imran Ullah designed the study, provided funds and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Ethical approval
The research study was approved by Institutional Review Board (IRB), Quaid-i-Azam University, Islamabad, Pakistan under ethical committee approval number ‘IRB-QA-176’. Informed written consent was taken from participating members of families. Blood sampling from normal and affected individuals were carried out according to guidelines provided by Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fayyaz, H., Zaman, A., Shabbir, S. et al. Mutational analysis in different genes underlying severe combined immunodeficiency in seven consanguineous Pakistani families. Mol Biol Rep 51, 302 (2024). https://doi.org/10.1007/s11033-024-09222-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09222-0