Abstract
Background
Lung cancer, one of the most common oncological diseases worldwide, continues to be the leading cause of cancer-related deaths. The development of new approaches for lung cancer, which still has a low survival rate despite medical advances, is of great importance.
Methods and results
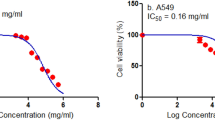
In this study, bee venom (BV), conditioned medium of MSCs isolated from dental follicles (MSC-CM) and cisplatin were applied at different doses and their effects on A549 cell line were evaluated. Dental follicles were used as a source of MSCs source and differentiation kits, and characterization studies (flow cytometry) were performed. Cell viability was measured by the MTT method and apoptosis was measured by an Annexin V-FITC/PI kit on flow cytometer. IC50 dose values were determined according to the 24th hour and were determined as 15.8 µg/mL for BV, 10.78% for MSC-CM and 5.77 µg/mL for cisplatin. IC50 values found for BV and MSC-CM were also given in combination and the effects were observed. It was found that the applied substances caused BV to decrease in cell viability and induced apoptosis in cells. In addition to the induction of apoptosis in BV, MSC-CM, and combined use, all three applications led to an increase in Bax protein expression and a decrease in Bcl-2 protein expression. The molecular mechanism of anticancer activity through inhibition of Bax and Bcl-2 proteins and the NF-κB signaling pathway may be suggested.
Conclusion
Isolated MSCs in our study showed anticancer activity and BV and MSC-CM showed synergistic antiproliferative and apoptotic effects.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
GLOBOCAN 2020. IARC World Health Organization. November 20 edn., https://gco.iarc.fr/today
Zappa C, Mousa SA (2016) Non-small cell lung cancer: current treatment and future advances. Trans Lung Cancer Res 5:288
Gupta SL, Basu S, Soni V, Jaiswal RK (2022) Immunotherapy: an alternative promising therapeutic approach against cancers. Mol Biol Rep 49:9903–9913. https://doi.org/10.1007/s11033-022-07525-8
Falzone L, Salomone S, Libra M (2018) Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol 9:1300. https://doi.org/10.3389/fphar.2018.01300
Mohr A, Zwacka R (2018) The future of mesenchymal stem cell-based therapeutic approaches for cancer-from cells to ghosts. Cancer Lett 414:239–249. https://doi.org/10.1016/j.canlet.2017.11.025
Renesme L, Pierro M, Cobey KD, Mital R, Nangle K, Shorr R, Lalu MM, Thébaud B (2022) Definition and characteristics of mesenchymal stromal cells in preclinical and clinical studies: a scoping review. Stem Cells Transl Med 11:44–54. https://doi.org/10.1093/stcltm/szab009
Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L (2019) Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT®) Mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy 21:1019–1024. https://doi.org/10.1016/j.jcyt.2019.08.002
Gan L, Liu Y, Cui D, Pan Y, Zheng L, Wan M (2020) Dental tissue-derived human mesenchymal stem cells and their potential in therapeutic application. Stem Cells Int 2020:8864572. https://doi.org/10.1155/2020/8864572
Vakhshiteh F, Atyabi F, Ostad SN (2019) Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomed 14:2847–2859. https://doi.org/10.2147/ijn.S200036
Linero I, Chaparro O (2014) Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 9:e107001. https://doi.org/10.1371/journal.pone.0107001
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9:63. https://doi.org/10.1186/s13287-018-0791-7
Kolayli S, Keskin M (2020) Natural bee products and their apitherapeutic applications. Stud Nat Prod Chem 66:175–196
Rady I, Siddiqui IA, Rady M, Mukhtar H (2017) Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett 402:16–31
Hwang DS, Kim SK, Bae H (2015) Therapeutic effects of bee venom on immunological and neurological diseases. Toxins (Basel) 7:2413–2421. https://doi.org/10.3390/toxins7072413
Wehbe R, Frangieh J, Rima M, El Obeid D, Sabatier JM, Fajloun Z (2019) Bee venom: overview of main compounds and bioactivities for therapeutic interests. Molecules. https://doi.org/10.3390/molecules24162997
Yaacoub C, Rifi M, El-Obeid D, Mawlawi H, Sabatier JM, Coutard B, Fajloun Z (2021) The cytotoxic effect of apis mellifera venom with a synergistic potential of its two main components-melittin and PLA2-on colon cancer HCT116 cell lines. Molecules. https://doi.org/10.3390/molecules26082264
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G (2018) molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a006080
Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J (2022) The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol 12:985363. https://doi.org/10.3389/fonc.2022.985363
Genç D, Zibandeh N, Nain E, Gökalp M, Özen AO, Göker MK, Akkoç T (2018) Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin Exp Allergy 48:663–678. https://doi.org/10.1111/cea.13126
de Silva M, Itchins M, Pavlakis N (2020) Breakthrough 5-year survival with pembrolizumab in Keynote-001 study: horizon shifting in advanced non-small cell lung cancer with immune check point inhibition. Ann Transl Med 8:555. https://doi.org/10.21037/atm.2020.01.87
Ranasinghe R, Mathai ML, Zulli A (2022) Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon 8:e10608. https://doi.org/10.1016/j.heliyon.2022.e10608
Sobral F, Sampaio A, Falcão S, Queiroz MJ, Calhelha RC, Vilas-Boas M, Ferreira IC (2016) Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem Toxicol 94:172–177. https://doi.org/10.1016/j.fct.2016.06.008
Frangieh J, Salma Y, Haddad K, Mattei C, Legros C, Fajloun Z, El Obeid D (2019) First characterization of the venom from Apis mellifera syriaca. A Honeybee from The Middle East Region Toxins (Basel). https://doi.org/10.3390/toxins11040191
Wang J, Li F, Tan J, Peng X, Sun L, Wang P, Jia S, Yu Q, Huo H, Zhao H (2017) Melittin inhibits the invasion of MCF-7 cells by downregulating CD147 and MMP-9 expression. Oncol Lett 13:599–604. https://doi.org/10.3892/ol.2016.5516
Jang MH, Shin MC, Lim S, Han SM, Park HJ, Shin I, Lee JS, Kim KA, Kim EH, Kim CJ (2003) Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J Pharmacol Sci 91:95–104. https://doi.org/10.1254/jphs.91.95
Zheng J, Lee HL, Ham YW, Song HS, Song MJ, Hong JT (2015) Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 6:44437–44451. https://doi.org/10.18632/oncotarget.6295
Choi KE, Hwang CJ, Gu SM, Park MH, Kim JH, Park JH, Ahn YJ, Kim JY, Song MJ, Song HS, Han SB, Hong JT (2014) Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins (Basel) 6:2210–2228. https://doi.org/10.3390/toxins6082210
Yu R, Wang M, Wang M, Han L (2020) Melittin suppresses growth and induces apoptosis of non-small-cell lung cancer cells via down-regulation of TGF-β-mediated ERK signal pathway. Braz J Med Biol Res 54:e9017. https://doi.org/10.1590/1414-431x20209017
Jeong YJ, Park YY, Park KK, Choi YH, Kim CH, Chang YC (2019) Bee venom suppresses EGF-induced epithelial-mesenchymal transition and tumor invasion in lung cancer cells. Am J Chin Med 47:1869–1883. https://doi.org/10.1142/s0192415x19500952
Zhang SF, Chen Z (2017) Melittin exerts an antitumor effect on non-small cell lung cancer cells. Mol Med Rep 16:3581–3586. https://doi.org/10.3892/mmr.2017.6970
Zhao J, Hu W, Zhang Z, Zhou Z, Duan J, Dong Z, Liu H, Yan C (2022) Bee venom protects against pancreatic cancer via inducing cell cycle arrest and apoptosis with suppression of cell migration. J Gastrointest Oncol 13:847–858. https://doi.org/10.21037/jgo-22-222
Huang Z, Tong Y, Wang J, Huang Y (2003) NMR studies of the relationship between the changes of membrane lipids and the cisplatin-resistance of A549/DDP cells. Cancer Cell Int 3:5. https://doi.org/10.1186/1475-2867-3-5
Dai CH, Li J, Chen P, Jiang HG, Wu M, Chen YC (2015) RNA interferences targeting the fanconi anemia/BRCA pathway upstream genes reverse cisplatin resistance in drug-resistant lung cancer cells. J Biomed Sci 22:77. https://doi.org/10.1186/s12929-015-0185-4
Baghaei K, Hashemi SM, Tokhanbigli S, Asadi Rad A, Assadzadeh-Aghdaei H, Sharifian A, Zali MR (2017) Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench 10:208–213
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B (2017) Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. https://doi.org/10.3390/ijms18071450
Luo D, Hu S, Tang C, Liu G (2018) Mesenchymal stem cells promote cell invasion and migration and autophagy-induced epithelial-mesenchymal transition in A549 lung adenocarcinoma cells. Cell Biochem Funct 36:88–94. https://doi.org/10.1002/cbf.3320
Wang X, Gao JL, Zhao MM, Zhu HX, Tian YX, Li R, Jiang XH, Yu L, Tian JR, Cui JZ (2018) Therapeutic effects of conditioned medium from bone marrow-derived mesenchymal stem cells on epithelial-mesenchymal transition in A549 cells. Int J Mol Med 41:659–668. https://doi.org/10.3892/ijmm.2017.3284
Lin W, Huang L, Li Y, Fang B, Li G, Chen L, Xu L (2019) Mesenchymal stem cells and cancer: clinical challenges and opportunities. Biomed Res Int 2019:2820853. https://doi.org/10.1155/2019/2820853
Lu L, Chen G, Yang J, Ma Z, Yang Y, Hu Y, Lu Y, Cao Z, Wang Y, Wang X (2019) Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed Pharmacother 112:108625. https://doi.org/10.1016/j.biopha.2019.108625
Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, Weiss M, Tamura M, Troyer D (2009) Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res 69:1815–1820. https://doi.org/10.1158/0008-5472.Can-08-2750
Li L, Pan J, Cai X, Gong E, Xu C, Zheng H, Cao Z, Yin Z (2020) Human umbilical cord mesenchymal stem cells suppress lung cancer via TLR4/NF-κB signalling pathway. Biotechnol Biotechnol Equip 34:24–29
Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD (2008) NF-kappaB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin 29:333–340. https://doi.org/10.1111/j.1745-7254.2008.00751.x
François S, Usunier B, Forgue-Lafitte ME, L’Homme B, Benderitter M, Douay L, Gorin NC, Larsen AK, Chapel A (2019) Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Transl Med 8:285–300. https://doi.org/10.1002/sctm.18-0117
Ryu H, Oh JE, Rhee KJ, Baik SK, Kim J, Kang SJ, Sohn JH, Choi E, Shin HC, Kim YM, Kim HS, Bae KS, Eom YW (2014) Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-β and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett 352:220–227. https://doi.org/10.1016/j.canlet.2014.06.018
Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80:267–274. https://doi.org/10.1016/j.yexmp.2005.07.004
Gazdic M, Simovic Markovic B, Jovicic N, Misirkic-Marjanovic M, Djonov V, Jakovljevic V, Arsenijevic N, Lukic ML, Volarevic V (2017) Mesenchymal stem cells promote metastasis of lung cancer cells by downregulating systemic antitumor immune response. Stem Cells Int 2017:6294717. https://doi.org/10.1155/2017/6294717
Doğan A, Demirci S, Apdik H, Apdik EA, Şahin F (2017) Dental pulp stem cells (DPSCs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue Cell 49:711–718. https://doi.org/10.1016/j.tice.2017.10.003
Acknowledgements
We would like to thank Selcuk University Scientific Research Projects Coordination Office and The Scientific and Technological Research Council of Türkiye (TUBITAK). We also thank Muslu Kazim Korez for statistical support.
Funding
This research was supported by Selçuk University Scientific Research Projects Coordination Office (Project No: 21112006) and The Scientific and Technological Research Council of Türkiye (TUBITAK) 1002 Short Term R&D Funding Program (Project No: 222S178).
Author information
Authors and Affiliations
Contributions
FS performed all the experiments, data analysis and drafting the manuscript. HV was supervised the project, editing the manuscript and participated in the finalization of the manuscript. BO participated in statistical analysis, interpretation of the results and editing the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was approved by Selcuk University Faculty of Medicine Ethics Committee (Number: 2021/05, Date: 10.03.2021).
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sengul, F., Vatansev, H. & Ozturk, B. Investigation the effects of bee venom and H-dental-derived mesenchymal stem cells on non-small cell lung cancer cells (A549). Mol Biol Rep 51, 2 (2024). https://doi.org/10.1007/s11033-023-09002-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09002-2