Abstract
Background
Glutamate exposure was fatal to HT-22 neuronal cells that derived from mouse hippocampus. This is often used as a model for hippocampus neurodegeneration in vitro. The targets relevant to glutamate-induced neuronal toxicity is not fully understood. In this study, we aimed to identify crucial factors associated with glutamate-induced cytotoxicity in HT-22 cells.
Methods
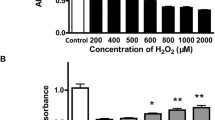
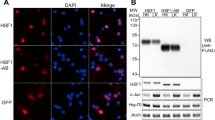
HT-22 cells were treated with 7.5 mM glutamate for 24 h and isobaric tags for relative and absolute quantitation (iTRAQ) proteomic analysis conducted to identify the differentially expressed proteins. Differential proteins were subjected to Gene Ontology analyses. Upregulation of barrier to autointegration factor (BANF1/BANF1) protein was confirmed by RT-qPCR and western blotting. Cell viability was measured by CKK-8 and MTT assays. Cell apoptosis rates and intracellular reactive oxygen species (ROS) levels were detected using flow cytometry.
Results
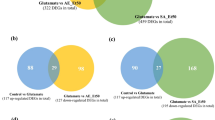
A total of 5811 proteins were quantified by iTRAQ, 50 of which were recognized as significantly differential proteins (fold change ≥ 1.5 and P ≤ 0.05); 26 proteins were up-regulated and 24 were down-regulated after exposure to glutamate. GO enrichment analysis showed that the apoptotic signaling pathway was involved in cell death induced by glutamate. BANF1 expression level was markedly increased in HT-22 cells after glutamate treatment. Further, knockdown of BANF1 alleviated glutamate-mediated cell death with lower ROS levels.
Conclusions
In conclusion, we successfully filtered out differential proteins relevant to glutamate-mediated cytotoxicity. BANF1 upregulation promoted glutamate-induced apoptosis of HT-22 cells by enhancing ROS generation.




Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request privately.
References
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hagg S, Athanasiu L, Voyle N, Proitsi P, Witoelar A, Stringer S, Aarsland D, Almdahl IS, Andersen F, Bergh S, Bettella F, Bjornsson S, Braekhus A, Brathen G, de Leeuw C, Desikan RS, Djurovic S, Dumitrescu L, Fladby T, Hohman TJ, Jonsson PV, Kiddle SJ, Rongve A, Saltvedt I, Sando SB, Selbaek G, Shoai M, Skene NG, Snaedal J, Stordal E, Ulstein ID, Wang Y, White LR, Hardy J, Hjerling-Leffler J, Sullivan PF, van der Flier WM, Dobson R, Davis LK, Stefansson H, Stefansson K, Pedersen NL, Ripke S, Andreassen OA, Posthuma D (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 51:404–413. https://doi.org/10.1038/s41588-018-0311-9
Fish PV, Steadman D, Bayle ED, Whiting P (2019) New approaches for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 29:125–133. https://doi.org/10.1016/j.bmcl.2018.11.034
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab, Clinical Trial I (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333. https://doi.org/10.1056/NEJMoa1304839
Antzoulatos EG, Byrne JH (2004) Learning insights transmitted by glutamate. Trends Neurosci 27:555–560. https://doi.org/10.1016/j.tins.2004.06.009
Smith AE, Kenyon DH (1973) A unifying concept of carcinogenesis and its therapeutic implications. Oncology 27:459–479. https://doi.org/10.1159/000224754
Chi H, Chang HY, Sang TK (2018) Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. https://doi.org/10.3390/ijms19103082
Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD (2015) Researching glutamate - induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci 9:91. https://doi.org/10.3389/fncel.2015.00091
Haraguchi T, Koujin T, Osakada H, Kojidani T, Mori C, Masuda H, Hiraoka Y (2007) Nuclear localization of barrier-to-autointegration factor is correlated with progression of S phase in human cells. J Cell Sci 120:1967–1977. https://doi.org/10.1242/jcs.03461
Halfmann CT, Sears RM, Katiyar A, Busselman BW, Aman LK, Zhang Q, O’Bryan CS, Angelini TE, Lele TP, Roux KJ (2019) Repair of nuclear ruptures requires barrier-to-autointegration factor. J Cell Biol 218:2136–2149. https://doi.org/10.1083/jcb.201901116
Jacque JM, Stevenson M (2006) The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature 441:641–645. https://doi.org/10.1038/nature04682
Samwer M, Schneider MWG, Hoefler R, Schmalhorst PS, Jude JG, Zuber J, Gerlich DW (2017) DNA cross-bridging shapes a single nucleus from a set of mitotic chromosomes. Cell 170(956–972):e923. https://doi.org/10.1016/j.cell.2017.07.038
Bolderson E, Burgess JT, Li J, Gandhi NS, Boucher D, Croft LV, Beard S, Plowman JJ, Suraweera A, Adams MN, Naqi A, Zhang SD, Sinclair DA, O’Byrne KJ, Richard DJ (2019) Barrier-to-autointegration factor 1 (Banf1) regulates poly [ADP-ribose] polymerase 1 (PARP1) activity following oxidative DNA damage. Nat Commun 10:5501. https://doi.org/10.1038/s41467-019-13167-5
Puente XS, Quesada V, Osorio FG, Cabanillas R, Cadinanos J, Fraile JM, Ordonez GR, Puente DA, Gutierrez-Fernandez A, Fanjul-Fernandez M, Levy N, Freije JM, Lopez-Otin C (2011) Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am J Hum Genet 88:650–656. https://doi.org/10.1016/j.ajhg.2011.04.010
Janssen A, Marcelot A, Breusegem S, Legrand P, Zinn-Justin S, Larrieu D (2022) The BAF A12T mutation disrupts lamin A/C interaction, impairing robust repair of nuclear envelope ruptures in Nestor-Guillermo progeria syndrome cells. Nucleic Acids Res 50:9260–9278. https://doi.org/10.1093/nar/gkac726
Takama H, Sugiura K, Ogawa Y, Muro Y, Akiyama M (2013) Possible roles of barrier-to-autointegration factor 1 in regulation of keratinocyte differentiation and proliferation. J Dermatol Sci 71:100–106. https://doi.org/10.1016/j.jdermsci.2013.04.007
Arendt T, Bruckner MK, Morawski M, Jager C, Gertz HJ (2015) Early neurone loss in Alzheimer’s disease: cortical or subcortical? Acta Neuropathol Commun 3:10. https://doi.org/10.1186/s40478-015-0187-1
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Pomara N, Singh R, Deptula D, Chou JC, Schwartz MB, LeWitt PA (1992) Glutamate and other CSF amino acids in Alzheimer’s disease. Am J Psychiatry 149:251–254. https://doi.org/10.1176/ajp.149.2.251
Berke JD, Sgambato V, Zhu PP, Lavoie B, Vincent M, Krause M, Hyman SE (2001) Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron 32:277–287. https://doi.org/10.1016/s0896-6273(01)00465-2
Yoneda Y, Kuramoto N, Kitayama T, Hinoi E (2001) Consolidation of transient ionotropic glutamate signals through nuclear transcription factors in the brain. Prog Neurobiol 63:697–719. https://doi.org/10.1016/s0301-0082(00)00036-8
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30:379–387. https://doi.org/10.1038/aps.2009.24
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2:1547–1558. https://doi.org/10.1016/0896-6273(89)90043-3
Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH (2007) Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem 103:2004–2014. https://doi.org/10.1111/j.1471-4159.2007.04884.x
Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, Kazhdan I, Becattini B, Zahler S, Vollmar A, Pellecchia M, Reichert A, Plesnila N, Wagner E, Culmsee C (2008) Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ 15:1553–1563. https://doi.org/10.1038/cdd.2008.78
Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C (2011) Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ 18:282–292. https://doi.org/10.1038/cdd.2010.92
Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11:e1001604. https://doi.org/10.1371/journal.pbio.1001604
Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R (2014) Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3:24722. https://doi.org/10.3402/jev.v3.24722
Verma M, Lizama BN, Chu CT (2022) Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl Neurodegener 11:3. https://doi.org/10.1186/s40035-021-00278-7
Koren SA, Hamm MJ, Meier SE, Weiss BE, Nation GK, Chishti EA, Arango JP, Chen J, Zhu H, Blalock EM, Abisambra JF (2019) Tau drives translational selectivity by interacting with ribosomal proteins. Acta Neuropathol 137:571–583. https://doi.org/10.1007/s00401-019-01970-9
Ding Q, Markesbery WR, Chen Q, Li F, Keller JN (2005) Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci 25:9171–9175. https://doi.org/10.1523/JNEUROSCI.3040-05.2005
Hernandez-Ortega K, Garcia-Esparcia P, Gil L, Lucas JJ, Ferrer I (2016) Altered machinery of protein synthesis in Alzheimer’s: from the nucleolus to the ribosome. Brain Pathol 26:593–605. https://doi.org/10.1111/bpa.12335
Suzuki M, Tezuka K, Handa T, Sato R, Takeuchi H, Takao M, Tano M, Uchida Y (2022) Upregulation of ribosome complexes at the blood-brain barrier in Alzheimer’s disease patients. J Cereb Blood Flow Metab 42:2134–2150. https://doi.org/10.1177/0271678X221111602
Kapp LD, Lorsch JR (2004) The molecular mechanics of eukaryotic translation. Annu Rev Biochem 73:657–704. https://doi.org/10.1146/annurev.biochem.73.030403.080419
Beckelman BC, Yang W, Kasica NP, Zimmermann HR, Zhou X, Keene CD, Ryazanov AG, Ma T (2019) Genetic reduction of eEF2 kinase alleviates pathophysiology in Alzheimer’s disease model mice. J Clin Invest 129:820–833. https://doi.org/10.1172/JCI122954
Kasica NP, Zhou X, Yang Q, Wang X, Yang W, Zimmermann HR, Holland CE, Koscielniak E, Wu H, Cox AO, Lee J, Ryazanov AG, Furdui CM, Ma T (2022) Antagonists targeting eEF2 kinase rescue multiple aspects of pathophysiology in Alzheimer’s disease model mice. J Neurochem 160:524–539. https://doi.org/10.1111/jnc.15562
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360. https://doi.org/10.1038/nrm.2017.20
Zhang M, Qian C, Zheng ZG, Qian F, Wang Y, Thu PM, Zhang X, Zhou Y, Tu L, Liu Q, Li HJ, Yang H, Li P, Xu X (2018) Jujuboside a promotes Abeta clearance and ameliorates cognitive deficiency in Alzheimer’s disease through activating Axl/HSP90/PPARgamma pathway. Theranostics 8:4262–4278. https://doi.org/10.7150/thno.26164
Koppula P, Zhang Y, Zhuang L, Gan B (2018) Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 38:12. https://doi.org/10.1186/s40880-018-0288-x
Montes de Oca R, Shoemaker CJ, Gucek M, Cole RN, Wilson KL (2009) Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS ONE 4:e7050. https://doi.org/10.1371/journal.pone.0007050
Rajagopalan S, Andreeva A, Teufel DP, Freund SM, Fersht AR (2009) Interaction between the transactivation domain of p53 and PC4 exemplifies acidic activation domains as single-stranded DNA mimics. J Biol Chem 284:21728–21737. https://doi.org/10.1074/jbc.M109.006429
Batta K, Kundu TK (2007) Activation of p53 function by human transcriptional coactivator PC4: role of protein-protein interaction, DNA bending, and posttranslational modifications. Mol Cell Biol 27:7603–7614. https://doi.org/10.1128/MCB.01064-07
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS (2002) BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849. https://doi.org/10.1038/ncb866
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62. https://doi.org/10.1038/nature14344
Funding
This study was funded by the Discipline Construction Project of Guangdong Medical University (4SG21229GDGFY01), the Science and Technology Program of Guangzhou (202103000051), and the Guangdong Provincial Natural Science Foundation General Project (2021A1515010780).
Author information
Authors and Affiliations
Contributions
XY and KH performed the experiments and analyzed the data. XY and KH designed the study. XY and XY drafted the manuscript. SD and ZY supervised the study, reviewed and edited the manuscript. XY and XY contributed equally to this work and share first authorship.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study does not require ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, X., Xu, X., Hu, K. et al. BANF1 promotes glutamate-induced apoptosis of HT-22 hippocampal neurons. Mol Biol Rep 50, 9441–9452 (2023). https://doi.org/10.1007/s11033-023-08889-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08889-1