Abstract
Background
Diabetic cardiomyopathy (DCM) is a form of cardiac dysfunction caused by diabetes, increasing heart failure and death. Studies shown that hyperglycemia-induced oxidative stress significantly affects heart structure and functional changes during diabetic cardiomyopathy. Fucoidans are sulfated polysaccharide derived from naturally available seaweeds and reported for various biological functions such as antioxidant, anti-diabetic, and anti-inflammatory. However, the therapeutic potential of Indian seaweeds against DCM remains largely unexplored. Therefore, the current study aimed to work on the cardioprotective effect of extracted fucoidan from Sargassum wightii (SwF) in alloxan-induced DCM.
Methods and results
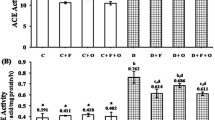
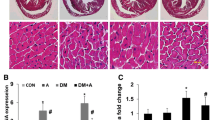
Diabetes (DM) was induced with alloxan monohydrate (150 mg/kg−1) dissolved in Nacl (0.9%) overnight–fasted rats. Group III, IV rats were DM induced, followed by treated with SwF (150 mg/kg−1) and (300 mg/kg−1). Group V and VI were non-diabetic rats and received SwF (150 mg/kg−1) and (300 mg/kg−1). SwF reduced classical progressive DM complications such as hyperglycemia, polydipsia, polyphagia, and polyurea in alloxan-induced diabetic rats. Biochemical analysis showed that SwF decreased blood glucose, cardiac markers enzymes, and lipid peroxidation levels compared to diabetic rats. SwF administration significantly increased Nrf2, HO-1, SOD, Catalase, and NQO1 gene expression. In addition, SwF-treated rats showed reduced heart tissue damage with increased Nrf2 and HO-1 protein expression.
Conclusion
The current research concludes that targeting oxidative stress with SwF provided an effective role in the prevention of DCM. Thus, fucoidan could be used to develop functional food ingredients for DCM.
Graphical abstract





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
References
Dillmann WH (2019) Diabetic cardiomyopathy: what is it and can it be fixed? Circul Res 124:1160–1162. https://doi.org/10.1161/CIRCRESAHA.118.314665
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122:624–638. https://doi.org/10.1161/CIRCRESAHA.117.311586
Booz GW, Baker KM (1995) Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res 30:537–543
Isfort M, Stevens SC, Schaffer S, Jong CJ, Wold LE (2014) Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev 19:35–48. https://doi.org/10.1007/s10741-013-9377-8
De Geest B, Mishra M (2022) Role of oxidative stress in diabetic cardiomyopathy. Antioxid (Basel 11:784. https://doi.org/10.3390/antiox11040784
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181–H2190. https://doi.org/10.1152/ajpheart.00554.2011
Ge Z-D, Lian Q, Mao X, Xia Z (2019) Current status and challenges of NRF2 as a potential therapeutic target for diabetic cardiomyopathy. Int Heart J 60:512–520. https://doi.org/10.1536/ihj.18-476
Parim B, Sathibabu Uddandrao VV, Saravanan G (2019) Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev 24:279–299. https://doi.org/10.1007/s10741-018-9749-1
Afonso NC, Catarino MD, Silva AMS, Cardoso SM (2019) Brown macroalgae as valuable food ingredients. Antioxid (Basel) 8:365. https://doi.org/10.3390/antiox8090365
Liu L, Heinrich M, Myers S, Dworjanyn SA (2012) Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J Ethnopharmacol 142:591–619. https://doi.org/10.1016/j.jep.2012.05.046
Ale MT, Meyer AS (2013) Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv 3:8131–8141. https://doi.org/10.1039/C3RA23373A
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695. https://doi.org/10.3390/molecules13081671
Ji Y, Jin D, Qi J, Wang X, Zhang C, An P, Luo Y, Luo J (2022) Fucoidan protects against doxorubicin-Induced cardiotoxicity by reducing oxidative stress and preventing mitochondrial function Injury. Int J Mol Sci. https://doi.org/10.3390/ijms231810685
Chang PM, Li KL, Lin YC (2019) Fucoidan(-)Fucoxanthin ameliorated cardiac function via IRS1/GRB2/ SOS1, GSK3beta/CREB pathways and metabolic pathways in senescent mice. Mar Drugs. https://doi.org/10.3390/md17010069
Yu X, Zhang Q, Cui W, Zeng Z, Yang W, Zhang C, Zhao H, Gao W, Wang X, Luo D (2014) Low molecular weight fucoidan alleviates cardiac dysfunction in diabetic Goto-Kakizaki rats by reducing oxidative stress and cardiomyocyte apoptosis. J Diabetes Res 2014:420929. https://doi.org/10.1155/2014/420929
Lin Z, Wang F, Yan Y, Jin J, Quan Z, Tong H, Du J (2023) Fucoidan derived from Sargassum pallidum alleviates metabolism disorders associated with improvement of cardiac injury and oxidative stress in diabetic mice. Phytother Res. https://doi.org/10.1002/ptr.7901
Chotigeat W, Tongsupa S, Supamataya K, Phongdara A (2004) Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 233:23–30. https://doi.org/10.1016/j.aquaculture.2003.09.025
Puhari SSM, Yuvaraj S, Vasudevan V, Ramprasath T, Rajkumar P, Arunkumar K, Amutha C, Selvam GS (2022) Isolation and characterization of fucoidan from four brown algae and study of the cardioprotective effect of fucoidan from Sargassum wightii against high glucose-induced oxidative stress in H9c2 cardiomyoblast cells. J Food Biochem 46:e14412. https://doi.org/10.1111/jfbc.14412
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Mahmoud AM (2017) Exercise amaliorates metabolic disturbances and oxidative stress in diabetic cardiomyopathy: possible underlying mechanisms. Adv Exp Med Biol 999:207–230. https://doi.org/10.1007/978-981-10-4307-9_12
Mohan M, Dihoum A, Mordi IR, Choy AM, Rena G, Lang CC (2021) Left ventricular hypertrophy in diabetic cardiomyopathy: a target for intervention. Front Cardiovasc Med 8:746382. https://doi.org/10.3389/fcvm.2021.746382
Ganesan K, Rana MBM, Sultan S (2018) Oral hypoglycemic medications. StatPearls Publishing, StatPearls
Saraswati, Giriwono PE, Iskandriati D, Andarwulan N (2021) Screening of In-Vitro anti-inflammatory and antioxidant activity of Sargassum ilicifolium crude lipid extracts from different coastal areas in Indonesia. Mar Drugs. https://doi.org/10.3390/md19050252
Zhang C, Jia J, Zhang P, Zheng W, Guo X, Ai C, Song S (2022) Fucoidan from Laminaria japonica ameliorates type 2 diabetes mellitus in association with modulation of gut microbiota and metabolites in streptozocin-treated mice. Foods. https://doi.org/10.3390/foods12010033
Wang X, Shan X, Dun Y, Cai C, Hao J, Li G, Cui K, Yu G (2019) Anti-metabolic syndrome effects of fucoidan from Fucus vesiculosus via reactive oxygen species-mediated regulation of JNK, akt, and AMPK signaling. Molecules. https://doi.org/10.3390/molecules24183319
Ren D, Wang Q, Yang Y, Hu Y, Song Y, He Y, Liu S, Wu L (2019) Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int J Biol Macromol 140:188–195. https://doi.org/10.1016/j.ijbiomac.2019.08.002
Mythili S, Malathi N (2015) Diagnostic markers of acute myocardial infarction. Biomed Rep 3:743–748. https://doi.org/10.3892/br.2015.500
Lekshmi VS, Rauf AA, Kurup GM (2019) Sulfated polysaccharides from the edible marine algae Padina tetrastromatica attenuates isoproterenol-induced oxidative damage via activation of PI3K/Akt/Nrf2 signaling pathway - an in vitro and in vivo approach. Chem Biol Interact 308:258–268. https://doi.org/10.1016/j.cbi.2019.05.044
Radenkovic M, Stojanovic M, Prostran M (2016) Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J Pharmacol Toxicol Methods 78:13–31. https://doi.org/10.1016/j.vascn.2015.11.004
Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L (2009) Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes 58:1391–1402. https://doi.org/10.2337/db08-1697
Abdel-Daim MM, Abushouk AI, Bahbah EI, Bungau SG, Alyousif MS, Aleya L, Alkahtani S (2020) Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ Sci Pollut Res Int 27:11554–11564. https://doi.org/10.1007/s11356-020-07711-w
Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7:385–394. https://doi.org/10.1089/ars.2005.7.385
Li B, Liu S, Miao L, Cai L (2012) Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. https://doi.org/10.1155/2012/216512
El-Omar MM, Yang ZK, Phillips AO, Shah AM (2004) Cardiac dysfunction in the Goto-Kakizaki rat. A model of type II diabetes mellitus. Basic Res Cardiol 99:133–141. https://doi.org/10.1007/s00395-004-0440-4
Acknowledgements
Shanavas Syed Mohamed Puhari expresses his gratitude to the UGC BSR fellowship program, New Delhi, India. The authors also thank, UGC-CEGS, UGC-CAS, UGC-NRCBS, DST PURSE and DST-FIST program for the central instrumentation facility at SBS, MKU.
Funding
The authors declare that no grants were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization—SSMP, GSS; Methodology—SSMP, SY, VV; Formal analysis—SSMP, TR, GSS; Investigation—SSMP, SY, VV; Writing - Original draft – SSMP, SY; Review and Editing – SSMP, SY, VV, TR, KA, CA and GSS; Funding acquisition – GSS.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The experimental procedure was approved by the Internal Research and Review Board, Ethical Clearance, Biosafety and Animal Welfare Committee of Madurai Kamaraj University.
Consent to publish
All authors have contributed significantly and agree with publication the content of the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puhari, S.S.M., Yuvaraj, S., Vasudevan, V. et al. Fucoidan from Sargassum wightii reduces oxidative stress through upregulating Nrf2/HO-1 signaling pathway in alloxan-induced diabetic cardiomyopathy rats. Mol Biol Rep 50, 8855–8866 (2023). https://doi.org/10.1007/s11033-023-08780-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08780-z