Abstract
Background
In HTLV-1-associated malignant disease, adult T-cell leukaemia/lymphoma (ATLL), the interaction of virus and host was evaluated at the chemokines gene expression level. Also, IL-1β and Caspase-1 expressions were evaluated to investigate the importance of pyroptosis in disease development and progression.
Methods and results
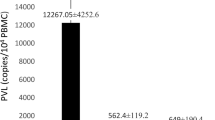
The expression of host CCR6 and CXCR-3 and the HTLV-1 proviral load (PVL), Tax, and HBZ were assessed in 17 HTLV-1 asymptomatic carriers (ACs) and 12 ATLL patients using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR), TaqMan method. Moreover, RT-qPCR, SYBR Green assay were performed to measure Caspase-1 and IL-1β expression. HTLV-1-Tax did not express in 91.5% of the ATLLs, while HBZ was expressed in all ATLLs. The expression of CXCR3 dramatically decreased in ATLLs compared to ACs (p = 0.001). The expression of CCR6 was lower in ATLLs than ACs (p = 0.04). The mean of PVL in ATLL patients was statistically higher than ACs (p = 0.001). Furthermore, the expression of the IL-1β between ATLLs and ACs was not statistically significant (p = 0.4). In contrast, there was a meaningful difference between Caspase-1 in ATLLs and ACs (p = 0.02).
Conclusions
The present study indicated that in the first stage of ATLL malignancy toward acute lymphomatous, CXCR3 and its progression phase may target the pyroptosis process. Mainly, HBZ expression could be a novel therapeutic target.





Similar content being viewed by others
Data Availability
All data supporting this study’s findings are included in the manuscript and available from the corresponding author upon reasonable request.
References
Gessain A, Cassar O (2012) Epidemiological aspects and World distribution of HTLV-1 infection. Front Microbiol 3:388. https://doi.org/10.3389/fmicb.2012.00388
Ahmadi Ghezeldasht S, Shirdel A, Assarehzadegan MA, Hassannia T, Rahimi H, Miri R et al (2013) Human T lymphotropic virus type I (HTLV-I) oncogenesis: molecular aspects of virus and host interactions in pathogenesis of adult T cell Leukemia/Lymphoma (ATL). Iran J Basic Med Sci 16(3):179–195
Mota TM, Jones RB (2019) HTLV-1 as a model for virus and host coordinated immunoediting. Front Immunol 10:2259. https://doi.org/10.3389/fimmu.2019.02259
Lemoine FJ, Wycuff DR, Marriott SJ (2001) Transcriptional activity of HTLV-I Tax influences the expression of marker genes associated with cellular transformation. Dis Markers 17(3):129–137. https://doi.org/10.1155/2001/263567
Saito M, Matsuzaki T, Satou Y, Yasunaga J, Saito K, Arimura K et al (2009) In vivo, expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology 6:19. https://doi.org/10.1186/1742-4690-6-19
Ahmadi Ghezeldasht S, Blackbourn DJ, Mosavat A, Rezaee SA (2023) Pathogenicity and virulence of human T lymphotropic virus type-1 (HTLV-1) in oncogenesis: adult T-cell leukaemia/lymphoma (ATLL). Critical Reviews in Clinical Laboratory Sciences. :1–23. https://doi.org/10.1080/10408363.2022.2157791
Griffith JW, Sokol CL, Luster AD (2014) Chemokines and chemokine receptors: positioning cells for host defence and immunity. Annu Rev Immunol 32:659–702
Tang P, Wang JM (2018) Chemokines: the past, the present and the future. Cell Mol Immunol 15(4):295–298. https://doi.org/10.1038/cmi.2018.9
Clark-Lewis I, Mattioli I, Gong J-H, Loetscher P (2003) Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem 278(1):289–295. https://doi.org/10.1074/jbc.M209470200
Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Xing C et al (2019) The role of CXCR3 in neurological Diseases. Curr Neuropharmacol 17(2):142–150. https://doi.org/10.2174/1570159x15666171109161140
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J et al (2014) CBTRUS statistical report: primary brain and central nervous system tumours diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–63. https://doi.org/10.1093/neuonc/nou223
Rafatpanah H, Felegari M, Azarpazhooh MR, Vakili R, Rajaei T, Hampson I et al (2017) Altered expression of CXCR3 and CCR6 and their ligands in HTLV-1 carriers and HAM/TSP patients. J Med Virol 89(8):1461–1468. https://doi.org/10.1002/jmv.24779
Hashikawa K, Yasumoto S, Nakashima K, Arakawa F, Kiyasu J, Kimura Y et al (2014) Microarray analysis of gene expression by the microdissected epidermis and dermis in mycosis fungoides and adult T-cell leukaemia/lymphoma. Int J Oncol 45(3):1200–1208. https://doi.org/10.3892/ijo.2014.2524
Naito T, Yasunaga JI, Mitobe Y, Shirai K, Sejima H, Ushirogawa H et al (2018) Distinct gene expression signatures induced by viral transactivators of different HTLV-1 subgroups that confer a different risk of HAM/TSP. Retrovirology 15(1):72. https://doi.org/10.1186/s12977-018-0454-x
Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B et al (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181(12):8391–8401
Heo YJ, Choi S-E, Lee N, Jeon JY, Han SJ, Kim DJ et al (2020) CCL20 induced by visfatin in macrophages via the NF-κB and MKK3/6-p38 signalling pathways contributes to hepatic stellate cell activation. Mol Biol Rep 47(6):4285–4293. https://doi.org/10.1007/s11033-020-05510-7
Frick VO, Rubie C, Keilholz U, Ghadjar P (2016) Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: an overview. World J Gastroenterol 22(2):833–841. https://doi.org/10.3748/wjg.v22.i2.833
Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I (2020) CC chemokines in a tumour: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci 21(20):7619
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y et al (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457(7225):102–106. https://doi.org/10.1038/nature07623
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related Inflamm Nat 454(7203):436–444
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T et al (2022) Role of pyroptosis in inflammation and cancer. Cell Mol Immunol 19(9):971–992
Shi J, Gao W, Shao F, Pyroptosis (2017) Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Hu Y, Wang B, Li S, Yang S (2022) Pyroptosis, and its role in central nervous system disease. J Mol Biol 434(4):167379
Wu Y, Zhang J, Yu S, Li Y, Zhu J, Zhang K et al (2022) Cell pyroptosis in health and inflammatory diseases. Cell death discovery 8(1):191
Derakhshan R, Mirhosseini A, Ahmadi Ghezeldasht S, Jahantigh HR, Mohareri M, Boostani R et al (2020) Abnormal vitamin D and lipid profile in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. Mol Biol Rep 47(1):631–637. https://doi.org/10.1007/s11033-019-05171-1
Ma G, Yasunaga J, Matsuoka M (2016) Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology 13:16. https://doi.org/10.1186/s12977-016-0249-x
Akbarin MM, Shirdel A, Bari A, Mohaddes ST, Rafatpanah H, Karimani EG et al (2017) Evaluation of the role of TAX, HBZ, and HTLV-1 proviral load on the survival of ATLL patients. Blood Res 52(2):106. https://doi.org/10.5045/br.2017.52.2.106
Satou Y, Matsuoka M (2012) Molecular and Cellular Mechanisms of Leukemogenesis of ATL: Emergent evidence of a significant role for HBZ in HTLV-1-Induced Pathogenesis. Leuk Res Treatment 2012:213653. https://doi.org/10.1155/2012/213653
Yamada K, Miyoshi H, Yoshida N, Shimono J, Sato K, Nakashima K et al (2021) Human T-cell lymphotropic virus HBZ and tax mRNA expression are associated with specific clinicopathological features in adult T-cell leukaemia/lymphoma. Mod Pathol 34(2):314–326. https://doi.org/10.1038/s41379-020-00654-0
Vandermeulen C, O’Grady T, Wayet J, Galvan B, Maseko S, Cherkaoui M et al (2021) The HTLV-1 viral oncoproteins tax and HBZ reprogram the cellular mRNA splicing landscape. PLoS Pathog 17(9):e1009919. https://doi.org/10.1371/journal.ppat.1009919
Kobayashi N, Konishi H, Sabe H, Shigesada K, Noma T, Honjo T et al (1984) Genomic structure of HTLV (human T-cell leukaemia virus): detection of the defective genome and its amplification in MT-2 cells. Embo j 3(6):1339–1343. https://doi.org/10.1002/j.1460-2075.1984.tb01974.x
Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S et al (2018) CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation–a target for novel cancer therapy. Cancer Treat Rev 63:40–47
Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM (2003) The CXC chemokine IP-10 stimulates HIV-1 replication. Virology 307(1):122–134
Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH (2009) Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis 200(11):1774–1780
Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y et al (2007) Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26(32):4679–4688
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109. https://doi.org/10.1038/nrmicro2070
Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O et al (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505(7484):509–514. https://doi.org/10.1038/nature12940
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y et al (2020) Pyroptosis: a new frontier in cancer. Biomed Pharmacother 121:109595
van Montfoort N, Olagnier D, Hiscott J (2014) Unmasking immune sensing of retroviruses: interplay between innate sensors and host effectors. Cytokine Growth Factor Rev 25(6):657–668. https://doi.org/10.1016/j.cytogfr.2014.08.006
Chu Q, Jiang Y, Zhang W, Xu C, Du W, Tuguzbaeva G et al (2016) Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget 7(51):84658–84665. https://doi.org/10.18632/oncotarget.12384
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X et al (2014) Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest 94(1):52–62. https://doi.org/10.1038/labinvest.2013.126
Sun Y, Guo Y (2018) Expression of Caspase-1 in breast cancer tissues and its effects on cell proliferation, apoptosis and invasion. Oncol Lett 15(5):6431–6435. https://doi.org/10.3892/ol.2018.8176
Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C et al (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A 107(50):21635–21640. https://doi.org/10.1073/pnas.1016814108
Acknowledgements
The results described in this paper were part of the student thesis. This study was subjected to MSc thesis in Medical Hematology and Medical Virology by M. Rahimzada and M. Nahavandi, respectively. The authors greatly thank the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Mashhad, Iran, for financially supporting the present study. We also are grateful to our kind colleagues in Immunology Research Center, Inflammation and Inflammatory Diseases Division, for their valuable help and great appreciation to the ATLL patients and ACs participating in this study.
Funding
This study was financially supported by the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Mashhad, Iran, under Grants [MUMS 971184 and MUMS 981133, recipient: SAR. Rezaee].
Author information
Authors and Affiliations
Contributions
Doing experiments: MR, MN and MS; Manuscript drafting and editing: AS, AM, NA and SAG; Research advisors: HS and NV; Research director, conception and design of the study; data analysis: SAR and MD. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest regarding this manuscript in other regions.
Ethics approval/Consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. The study was reviewed and approved by the Biomedical Research Ethics Committee of the Mashhad University of Medical Sciences [IR.MUMS.REC.971184 and IR.MUMS.REC.981133]. The written informed consent forms were obtained and signed by all the participants. All methods were performed following relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Nahavandi and M. Saffari are co-first authors of this manuscript and have equal credit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahimzada, M., Nahavandi, M., Saffari, M. et al. Gene expression study of host-human T-cell leukaemia virus type 1 (HTLV-1) interactions: adult T-cell leukaemia/lymphoma (ATLL). Mol Biol Rep 50, 7479–7487 (2023). https://doi.org/10.1007/s11033-023-08626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08626-8