Abstract
Background
Harmaline is a β-carboline alkaloid that can be extracted from the seeds of Peganum harmala. Harmaline has been shown to exhibit a potent cytotoxic effect against tumor cells. In this study, the anti-glioblastoma activity of harmaline was investigated in vitro.
Methods and results
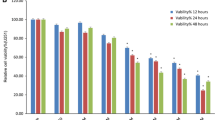
Cell viability, apoptosis, and cell cycle arrest were assessed in U-87 cells treated with harmaline at different doses. Reactive oxygen species (ROS) generation and the mRNA expression of apoptosis-associated genes were assessed. The anti-metastatic effect of harmaline on U-87 cells was evaluated by gelatin zymography assay where matrix metalloproteinase [MMP]-2/-9 enzymatic activity was measured, and the scratch assay was used to assess migratory responses. Flow cytometry demonstrated that harmaline could suppress the proliferation and induce sub-G1 cell cycle arrest and apoptotic cell death in glioblastoma cells. Harmaline treatment was also associated with an upregulation of the cell cycle-related genes, p21 and p53, and pro-apoptotic Bax, as well as the induction of ROS. The zymography assay indicated that the essential steps of metastasis were potently suppressed by harmaline through inhibiting the expression of MMP-2 and − 9. In addition, the migration of U-87 cells was significantly reduced after harmaline treatment.
Conclusion
Our data suggest a basis for further research of harmaline which has potential cytotoxic activities in glioblastoma cells; inducing cell cycle arrest and apoptosis, repression of migration, possibly invasion, and metastasis.





Similar content being viewed by others
Data Availability
The data supporting findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Tavana E et al (2020) Quercetin: a promising phytochemical for the treatment of glioblastoma multiforme. BioFactors 46(3):356–366
Fisher JP, Adamson DC (2021) Current FDA-Approved therapies for High-Grade Malignant Gliomas. Biomedicines 9(3):324
Seraj QM (2022) Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol Bio Rep
Khan FA et al (2013) Recent pharmacological developments in β-carboline alkaloid “harmaline”. Eur J Pharmacol 721(1):391–394
Rashidi M et al (2022) Harmaline downregulates angiogenesis markers and suppresses the growth of 4T1 breast cancer cells in vivo and in vitro. Chemico-Biol Interact 365:110087
β-Carboline Alkaloids: Biochemical and Pharmacological Functions Current Medicinal Chemistry, 2007. 14(4):479–500
Hilber P, Chapillon P (2005) Effects of harmaline on anxiety-related behavior in mice. Physiol Behav 86(1–2):164–167
Lamchouri F et al (2013) Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pak J Pharm Sci 26(4):699–706
Douer D (2002) New advances in the treatment of acute promyelocytic leukemia. Int J Hematol 76(Suppl 2):179–187
Zaker F, Oody A, Arjmand A (2007) A study on the antitumoral and differentiation effects of peganum harmala derivatives in combination with atra on leukaemic cells. Arch Pharm Res 30(7):844–849
Lamchouri F et al (2000) In vitro cell-toxicity of Peganum harmala alkaloids on cancerous cell-lines. Fitoterapia 71(1):50–54
Luo X-J, Peng J, Li Y-J (2011) Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 650(1):1–7
Arshad N et al (2008) Peganum harmala can minimize Escherichia coli infection in Poultry, but Long-Term Feeding May Induce Side Effects. Poult Sci 87(2):240–249
Astulla A et al (2008) Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med 62(4):470–472
Wang Y et al (2015) Novel mechanism of harmaline on inducing G2/M cell cycle arrest and apoptosis by up-regulating Fas/FasL in SGC-7901 cells. Sci Rep 5:18613
Hashemi S, Shabani S et al (2015) Peganum harmala L.’s anti-growth effect on a breast cancer cell line. Biotechnol Rep 8:138–143
Xu B et al (2018) Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol Lett 15(2):1931–1936
Jalili-Nik M et al (2020) Zerumbone Promotes Cytotoxicity in Human Malignant Glioblastoma Cells through Reactive Oxygen Species (ROS) Generation Oxid Med Cell Longev, 2020. 3237983
Sato H et al (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370(6484):61–65
Afshari AR et al (2020) Modulation of Calcium Signaling in Glioblastoma Multiforme: A Therapeutic Promise for Natural Products. Mini Rev Med Chem 20(18):1879–1899
Foroutan Z et al (2022) Plant-based synthesis of cerium oxide nanoparticles as a drug delivery system in improving the anticancer effects of free temozolomide in glioblastoma (U87) cells. Ceram Int 48(20):30441–30450
Roy S et al (2020) Discovery of Harmaline as a potent inhibitor of Sphingosine Kinase-1: a Chemopreventive Role in Lung Cancer. ACS Omega 5(34):21550–21560
Schedle A et al (1995) Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. J Dent Res 74(8):1513–1520
Gousias K, Theocharous T, Simon M (2022) Mech Cell Cycle Arrest Apoptosis Glioblastoma Biomedicines 10(3):564
Zhang Y et al (2021) Harmaline isolated from Peganum harmala suppresses growth of esophageal squamous cell carcinoma through targeting mTOR. Phytother Res 35(11):6377–6388
Hernández Borrero LJ, El-Deiry WS (2021) Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1876(1):188556
Das B, Sahoo S, Mallick B (2022) HIWI2 induces G2/M cell cycle arrest and apoptosis in human fibrosarcoma via the ROS/DNA damage/p53 axis. Life Sci 293:120353
Engeland K (2022) Cell cycle regulation: p53-p21-RB signaling. Cell Death & Differentiation 29(5):946–960
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities Nat Rev Cancer, 9(6):400–14
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1863:2977–299212
Siddiqui H et al (2017) Harmaline and its derivatives against the infectious Multi-Drug Resistant Escherichia coli. Med Chem 13(5):465–476
Li S-P et al (2018) Analogous β-Carboline alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Frontiers in Pharmacology, p 9
Paromita B et al (2018) Therapeutic potential of harmaline, a novel alkaloid, against cervical cancer cells in vitro: apoptotic induction and DNA interaction study. J Appl Biology Biotechnol 6(4):1–8
Ebrahimi S, Soltani A, Hashemy SI (2019) Oxidative stress in cervical cancer pathogenesis and resistance to therapy. J Cell Biochem 120(5):6868–6877
He Z, Simon HU (2013) A novel link between p53 and ROS. Cell Cycle 12(2):201–202
Jalili-Nik M et al (2019) Cytotoxic Effects of Ferula Latisecta on Human Glioma U87 cells. Drug Res (Stuttg) 69(12):665–670
Kumar R et al (2018) Demethoxycurcumin mediated targeting of MnSOD leading to activation of apoptotic pathway and inhibition of Akt/NF-κB survival signalling in human glioma U87 MG cells. Toxicol Appl Pharmacol 345:75–93
Hamsa TP, Kuttan G (2010) Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol 649(1–3):64–73
Funding
This study supported by Mashhad University of Medical Sciences (code: 4010356).
Author information
Authors and Affiliations
Contributions
AB: Methodology, Conceptualization, Formal analysis, Validation, Funding acquisition, Writing - original draft. MMV: Formal analysis, Validation, Writing - review & editing. AS: Methodology, Formal analysis, Validation. MM: Resources, Supervision, Writing - review & editing. SG: Methodology, Formal analysis, Validation. SASR: Validation, Writing - review & editing. GP: Resources, Supervision, Writing - review & editing. AA Methodology, Formal analysis, Validation. GF: Supervision, review & editing. All Authors read and confirmed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the Deputy of Research and Technology and Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1401.322).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ali Shahini and Mohammad Mahdi Vahedi are equal contributors as first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vahedi, M., Shahini, A., Mottahedi, M. et al. Harmaline exerts potentially anti-cancer effects on U-87 human malignant glioblastoma cells in vitro. Mol Biol Rep 50, 4357–4366 (2023). https://doi.org/10.1007/s11033-023-08354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08354-z