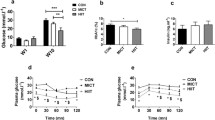
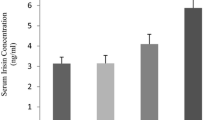
Abstract
Background
High-intensity Interval Training (HIIT) is a time-efficient form of exercise and has gained popularity in recent years. However, at molecular level, the understanding about the effects of HIIT is not comprehensive, and even less is elucidated about HIIT of different training duration cycles, although different durations always lead to different post-training consequences.
Method
In this study, by training SD rats using HIIT protocols lasting for different training duration cycles, we investigated the adaptive response of intramuscular triglyceride abundance as well as mitochondrial and lipid metabolic changes after HIIT training (2, 4, 6, 8, and 10 weeks). We selected 72 h after the last session of training as the time point of sacrifice.
Results
The suppressed activation of the cAMP-PKA pathway indicates that skeletal muscle was in the recovery phase at this time point. Intramuscular triglyceride abundance was significantly elevated after 2, 4, and 10 weeks of HIIT. However, the lipid metabolism-related proteins inconsistently changed in a chaotic trend (see Table 1). The expression levels of PGC1-α and COX IV decreased after 2 and 4 weeks of training and raised after 6 and 8 weeks of training. The expression level of citrate synthase (CS) decreased after 2, 4, 8, and 10 weeks of training, and showed an upward trend after 6 weeks of training. While the activity of CS decreased after 2 and 8 weeks of training and showed an upward trend after 6 weeks of HIIT.
Conclusion
Given the abovementioned changing trends, we propose two speculations: (A) the damaged mitochondria oxidation capacity might be one of the causes of IMTG accumulation observed after 2 and 4 weeks of HIIT. This phase might be similar to the condition of type 2 diabetes. (B) after 6-week HIIT, mitochondria function and biogenesis might be improved and the IMTG contents declined to baseline. This might be explained as: mitochondrial enhancement increased the capacity of lipid oxidation and then offset the increase in IMTG achieved during the first 4 weeks. For HIIT Rat Modelling, if the aim is to observe HIIT-induced positive effects, caution should be exercised when considering 2 and 4 weeks of training under our HIIT frame. Also, implementing six-week training is at least effective for mitochondrial enhancement when using similar HIIT frame of this study.






Similar content being viewed by others
Data availability
The supplement WB data used to support the findings of this study has been uploaded and is available from the corresponding author upon request.
References
Hargreaves M (2000) Skeletal muscle metabolism during exercise in humans. Clin Exp Pharmacol Physiol 27:225–228
Jesse S, Bayer H, Alupei MC, Zügel M, Mulaw M, Tuorto F, Malmsheimer S, Singh K, Steinacker J, Schumann U (2017) Ribosomal transcription is regulated by PGC-1alpha and disturbed in Huntington’s disease. Sci Rep 7:1–10
Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD (2009) Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol 106:1819–1825
Purdom T, Kravitz L, Dokladny K, Mermier C (2018) Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 15:1–10
van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH (2003) Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553:611–625
Martin W 3rd, Dalsky G, Hurley B, Matthews D, Bier D, Hagberg J, Rogers M, King D, Holloszy J (1993) Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol-Endocrinol Metabol 265:E708–E714
Romijn J, Coyle E, Sidossis L, Gastaldelli A, Horowitz J, Endert E, Wolfe R (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol-Endocrinol Metabol 265:E380–E391
Hwang J-H, Kim KM, Oh HT, Yoo GD, Jeong MG, Lee H, Park J, Jeong K, Kim YK, Ko Y-G (2022) TAZ links exercise to mitochondrial biogenesis via mitochondrial transcription factor A. Nat Commun 13:1–12
Blue MN, Smith-Ryan AE, Trexler ET, Hirsch KR (2018) The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J Sci Med Sport 21:207–212
Dubé JJ, Broskey NT, Despines AA, Stefanovic-Racic M, Toledo FG, Goodpaster BH, Amati F (2016) Muscle characteristics and substrate energetics in lifelong endurance athletes. Med Sci Sports Exerc 48:472
Mina C, Leia Z, Siyua C, Ruonanb S, Yaqiana Q, Jingquana S, Sun J (2021) Acute and chronic effects of high-intensity interval training (HIIT) on postexercise intramuscular lipid metabolism in rats. Physiol Res 70:735–743
Moreira JB, Bechara LR, Bozi LH, Jannig PR, Monteiro AW, Dourado PM, Wisløff U, Brum PC (2013) High-versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol 114:1029–1041
Schrauwen P, van Aggel-Leijssen DP, Hul G, Wagenmakers AJ, Vidal H, Saris WH, van Baak MA (2002) The effect of a 3-month low-intensity endurance training program on fat oxidation and acetyl-CoA carboxylase-2 expression. Diabetes 51:2220–2226
Shaw CS, Shepherd SO, Wagenmakers AJ, Hansen D, Dendale P, Van Loon LJ (2012) Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. Am J Physiol-Endocrinol Metabol 303:E1158–E1165
Eynon N, Ruiz JR, Meckel Y, Morán M, Lucia A (2011) Mitochondrial biogenesis related endurance genotype score and sports performance in athletes. Mitochondrion 11:64–69
Jacobs RA, Lundby C (2013) Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol 114:344–350
Zhang Q, Shen F, Shen W, Xia J, Wang J, Zhao Y, Zhang Z, Sun Y, Qian M, Ding S (2020) High-intensity interval training attenuates ketogenic diet-induced liver fibrosis in type 2 diabetic mice by ameliorating TGF-β1/Smad signaling. Diab Metabol Syndr Obes 13:4209
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588:1011–1022
Hoshino D, Yoshida Y, Kitaoka Y, Hatta H, Bonen A (2013) High-intensity interval training increases intrinsic rates of mitochondrial fatty acid oxidation in rat red and white skeletal muscle. Appl Physiol Nutr Metab 38:326–333
Wilk M, Krzysztofik M, Gepfert M, Poprzecki S, Gołaś A, Maszczyk A (2018) Technical and training related aspects of resistance training using blood flow restriction in competitive sport-a review. J Hum Kinet 65:249–260
Dudley GA, Abraham WM, Terjung RL (1982) Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53:844–850
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol-Regul Integr Comparative Physiol 266:R375–R380
Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Böhm M, Kindermann W, Nickenig G (2005) Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Prev Cardiol 12:407–414
Hamidie RDR, Yamada T, Ishizawa R, Saito Y, Masuda K (2015) Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 64:1334–1347
Wang YY, Pu XY, Shi WG, Fang QQ, Chen XR, Xi HR, Gao YH, Zhou J, Xian CJ, Chen KM (2019) Pulsed electromagnetic fields promote bone formation by activating the sAC–cAMP–PKA–CREB signaling pathway. J Cell Physiol 234:2807–2821
Yang H, Yang L (2016) Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J Mol Endocrinol 57:R93–R108
Park J, Kim S, Lee S, Jang H, Kim N, Lee S, Jung H, Kim I, Seong H, Choi B-H (2012) Effects of dietary fat types on growth performance, pork quality, and gene expression in growing-finishing pigs. Asian Australas J Anim Sci 25:1759
Shaik AH, Al-Omar SY, Mohammad A, Kodidhela LD (2020) Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci Rep 10:1–13
Bakhtiarizadeh MR, Salehi A, Alamouti AA, Abdollahi-Arpanahi R, Salami SA (2019) Deep transcriptome analysis using RNA-Seq suggests novel insights into molecular aspects of fat-tail metabolism in sheep. Sci Rep 9:1–14
Ito S (2019) High-intensity interval training for health benefits and care of cardiac diseases-the key to an efficient exercise protocol. World J Cardiol 11:171
Cortassa S, Sollott SJ, Aon MA (2017) Mitochondrial respiration and ROS emission during β-oxidation in the heart: an experimental-computational study. PLoS Comput Biol 13:e1005588
Yechoor VK, Patti M-E, Saccone R, Kahn CR (2002) Coordinated patterns of gene expression for substrate and energy metabolism in skeletal muscle of diabetic mice. Proc Natl Acad Sci 99:10587–10592
Strauss JA, Shepherd DA, Macey M, Jevons EF, Shepherd SO (2020) Divergence exists in the subcellular distribution of intramuscular triglyceride in human skeletal muscle dependent on the choice of lipid dye. Histochem Cell Biol 154:369–382
Modi S, Yaluri N, Kokkola T, Laakso M (2017) Plant-derived compounds strigolactone GR24 and pinosylvin activate SIRT1 and enhance glucose uptake in rat skeletal muscle cells. Sci Rep 7:1–11
MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ (2013) Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am J Physiol-Regul Integr Comparative Physiol 304:R644–R650
Chen S, Zhou L, Sun J, Qu Y, Chen M (2021) The role of cAMP-PKA pathway in lactate-induced intramuscular triglyceride accumulation and mitochondria content increase in mice. Front Physiol. https://doi.org/10.3389/fphys.2021.709135
Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, Nishida K, Sugimoto R, Nagao S, Miyamura S (2017) Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 8:735–747
Komiya Y, Sugiyama M, Ochiai M, Osawa N, Adachi Y, Iseki S, Arihara K (2021) Dietary olive oil intake improves running endurance with intramuscular triacylglycerol accumulation in mice. Nutrients 13:1164
Frøyland L, Madsen L, Vaagenes H, Totland G, Auwerx J, Kryvi H, Staels B, Berge R (1997) Mitochondrion is the principal target for nutritional and pharmacological control of triglyceride metabolism. J Lipid Res 38:1851–1858
Roden M (2005) Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes 29:S111–S115
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Sun J, Ye X, Xie M, Ye J (2016) Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep 6:1–10
Zhou L, Chen S, Han H, Sun J (2021) Lactate augments intramuscular triglyceride accumulation and mitochondrial biogenesis in rats. J Biol Regul Homeostat Agents 35:105–115
Mindikoglu AL, Abdulsada MM, Jain A, Jalal PK, Devaraj S, Wilhelm ZR, Opekun AR, Jung SY (2020) Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome. Sci Rep 10:1–14
Gao Z, Lai Q, Yang Q, Xu N, Liu W, Zhao F, Liu X, Zhang C, Zhang J, Jia L (2018) The characteristic, antioxidative and multiple organ protective of acidic-extractable mycelium polysaccharides by Pleurotus eryngii var. tuoliensis on high-fat emulsion induced-hypertriglyceridemic mice. Sci Rep 8:1–12
Zhang X, Wang C, Song G, Gan K, Kong D, Nie Q, Ren L (2013) Mitofusion-2-mediated alleviation of insulin resistance in rats through reduction in lipid intermediate accumulation in skeletal muscle. J Biomed Sci 20:1–9
Li Y, Wang J, Elzo MA, Gan M, Tang T, Shao J, Lai T, Ma Y, Jia X, Lai S (2021) Multi-omics analysis of key microRNA–mRNA metabolic regulatory networks in skeletal muscle of obese rabbits. Int J Mol Sci 22:4204
Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS (2014) Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc 46:1089
Walrand S, Short KR, Heemstra LA, Novak CM, Levine JA, Coenen-Schimke JM, Nair KS (2014) Altered regulation of energy homeostasis in older rats in response to thyroid hormone administration. FASEB J 28:1499–1510
Balampanis K, Chasapi A, Kourea E, Tanoglidi A, Hatziagelaki E, Lambadiari V, Dimitriadis G, Lambrou GI, Kalfarentzos F, Melachrinou M (2019) Inter-tissue expression patterns of the key metabolic biomarker PGC-1α in severely obese individuals: implication in obesity-induced disease. Hellenic J Cardiol 60:282–293
Kadenbach B (2021) Complex IV–The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion 58:296–302
Huang Y, Lü X, Chen R, Chen Y (2020) Comparative study of the effects of gold and silver nanoparticles on the metabolism of human dermal fibroblasts. Regen Biomater 7:221–232
Nederlof R, van den Elshout MA, Koeman A, Uthman L, Koning I, Eerbeek O, Weber NC, Hollmann MW, Zuurbier CJ (2017) Cyclophilin D ablation is associated with increased end-ischemic mitochondrial hexokinase activity. Sci Rep 7:1–13
Gil CI, Ost M, Kasch J, Schumann S, Heider S, Klaus S (2019) Role of GDF15 in active lifestyle induced metabolic adaptations and acute exercise response in mice. Sci Rep 9:1–9
Wang Y, Kang Y, Qi C, Zhang T, Zhao H, Ji X, Yan W, Huang Y, Cui R, Zhang G (2020) Pentoxifylline enhances antioxidative capability and promotes mitochondrial biogenesis for improving age-related behavioral deficits. Aging (Albany NY) 12:25487
Ratner C, Madsen AN, Kristensen LV, Skov LJ, Pedersen KS, Mortensen OH, Knudsen GM, Raun K, Holst B (2015) Impaired oxidative capacity due to decreased CPT1b levels as a contributing factor to fat accumulation in obesity. Am J Physiol-Regul Integr Comparatice Physiol 308:R973–R982
Joseph JS, Ayeleso AO, Mukwevho E (2017) Role of exercise-induced calmodulin protein kinase (CAMK) II activation in the regulation of omega-6 fatty acids and lipid metabolism genes in rat skeletal muscle. Physiol Res 66:969–977
Zhou L-G, Zhou X-H, Xu X-K, Liang Y-L, Gao F, Zhang C, Sun L-H, Ma X-S (2017) Experimental study on the effect of moxibustion at Shenque (CV 8) for long-term exercise-induced fatigue. J Acupuncture Tunia Sci 15:387–391
Zhang Y, Wen L, Nie J (2000) Study on molecular mechanism of exercise-induced fatigue in mitochondrial membrane III: relationships between proton potential energy across membrane and generation of free radicals during acute exercise. Chin J Sports Med 19:346–349
Liu L, Zhang Y, Liu T, Ke C, Huang J, Fu Y, Lin Z, Chen F, Wu X, Chen Q (2021) Pyrroloquinoline quinone protects against exercise-induced fatigue and oxidative damage via improving mitochondrial function in mice. FASEB J 35:e21394
Ostojic SM (2016) Exercise-induced mitochondrial dysfunction: a myth or reality? Clin Sci 130:1407–1416
Layec G, Blain GM, Rossman MJ, Park SY, Hart CR, Trinity JD, Gifford JR, Sidhu SK, Weavil JC, Hureau TJ (2018) Acute high-intensity exercise impairs skeletal muscle respiratory capacity. Med Sci Sports Exerc 50:2409
Loy BD, O’Connor PJ, Dishman RK (2016) Effect of acute exercise on fatigue in people with ME/CFS/SEID: a meta-analysis. Med Sci Sports Exerc 48:2003
Acknowledgements
Thanks to all the peer reviewers for their opinions and suggestions.
Funding
This work was funded by a grant from the National Natural Science Foundation of China (No. 31801002). The authors are appreciative for Dr. Jingquan Sun’s help in obtaining the education grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The paper is original and has not been published and is not being considered for publication elsewhere. All procedures in the present study were approved by the Sichuan University animal ethics committee and carried out according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shangguan, R., Hu, Z., Luo, Y. et al. Intramuscular mitochondrial and lipid metabolic changes of rats after regular high-intensity interval training (HIIT) of different training periods. Mol Biol Rep 50, 2591–2601 (2023). https://doi.org/10.1007/s11033-022-08205-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08205-3