Abstract
Background
Kazal-type serine protease inhibitors play a role in physiological processes such as blood coagulation and fibrinolysis. The amino acid residues at the P1 site are different, and they inhibit different types of proteases. The inhibitory mechanism of the protease in the salivary glands of Poecilobdella manillensis is still unclear.
Methods and results
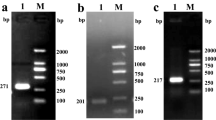
Based on cloning, prokaryotic expression and bioinformatics analysis, we studied the role of Kazal-type serine protease inhibitors in P. manillensis and analyzed their expression by quantitative real-time PCR. The results suggested that the recombinant protein was successfully expressed in the supernatant when a prokaryotic expression vector was constructed and induced with 0.2 mmol/L IPTG at 37 °C for 4 h, and the enzymatic activity was determined. The mature protein encodes 91 amino acids and has a relative molecular weight of 9929.32 Da, and after removing the signal peptide, the theoretical isoelectric point was 8.79. It is an unstable protein without a transmembrane domain. The mature protein contains two Kazal-type domains, in which all P1 residues are Lys, consisting of an α helix and three antiparallel β sheets. The upregulated expression of the mRNA was induced after a meal was provided, and the results showed an increasing and then decreasing trend.
Conclusions
Taken together, the results indicate that mature proteins from P. manillensis inhibit thrombin activity, laying the foundation for the subsequent in-depth study of the function of genes encoding Kazal-type serine protease inhibitors.
Highlights
-
The pmKPI cDNA from salivary glands of Poecilobdella manillensis was cloned and expressed.
-
pmKPI proteins can inhibit thrombin activity.
-
pmKPI mRNA expression was upregulated after a meal was provided, showing an increasing and then decreasing trend.





Similar content being viewed by others
Abbreviations
- p. manillensis :
-
Poecilobdella manillensis
- pmKPI :
-
Poecilobdella manillensis Kazal-type serine protease inhibitors
- KPI :
-
Kazal-type serine protease inhibitors
- SPIs :
-
Serine protease inhibitors
- Qpcr :
-
Quantitative Real-time-PCR
- H. medicinalis :
-
Hirudo medicinalis
- H. nipponica :
-
Hirudo nipponica
References
Shang Z-Y (2008) Annotation of Shennong herbal Scripture. Academe, Beijing
Tao H (1986) MingYi BieLu (Collect and criticism version). People’s Medical Publishing House, Beijing
Yu M, Zhou M, Cao M et al (2021) Review on the Development of the Medicinal Leech. J Aquaculture 42:39–43
Han D, Ren J-X, Wang J-J et al (2016) Chinese medicinal leech: ethnopharmacology, phytochemistry, and pharmacological activities. Evidence-Based Complementray and Alternative Medicine 2016:1–11. https://doi.org/10.1155/2016/7895935
Zhang W, Zhang R-X, Li J et al (2013) Species study on Chinese medicine leech and discussion on its resource sustainable utilization. China J Chin Materia Med 38:914–918. https://doi.org/10.4268/cjcmm20130629
Wei H, Li M, Duan H-Y et al (2015) Study on anti-thrombin activity of poecilobdella manillensis freezing dried powder with different reagents treatment. J Chengdu Univ Traditional Chin Med 38:37–40. https://doi.org/10.13593/j.cnki.51-1501/r.2015.01.037
Alessandro V, Vincenzo D-F, Giuseppe Z et al (1995) Probing the Structure of Hirudin from Hirudinaria manillensis by Limited Proteolysis. Eur J Biochem 226:323–333. https://doi.org/10.1111/j.1432-1033.1994.tb20056.x
Huang A-M, Li Z-Y, Liao G-S et al (2006) Purification of anticoagulation protein from the digestive juice of Guangxi hirudinaria manillensis. Chin J Biochem Pharm 27:273–276. https://doi.org/10.1038/sj.cr.7310110
Scacheri E, Nitti G, Valsasina B et al (2010) Novel hirudin variants from the leech Hirudinaria manillensis. Amino acid sequence, cDNA cloning and genomic organization. FEBS J 214:295–304. https://doi.org/10.1111/j.1432-1033.1993.tb17924.x
Guan D-L, Yang J, Liu Y-K et al (2019) Draft Genome of the Asian Buffalo Leech Hirudinaria manillensis. Front Genet 10:1321–1321. https://doi.org/10.3389/fgene.2019.01321
Liu Y-Y, Jiang S, Li Q et al (2021) Advances of Kunitz-type serine protease inhibitors. Chin J Biotechnol 37:3988–4000. https://doi.org/10.13345/j.cjb.200802
Law RH, Zhang Q-W, McGowan S et al (2006) An overview of the serpin superfamily. Genome Biol 7:216. https://doi.org/10.1186/gb-2006-7-5-216
Shakeel M, Xu X, Mandal SD et al (2019) Role of serine protease inhibitors in insect-host-pathogen interactions[J]. Arch Insect Biochem Physiol 102:e21556. https://doi.org/10.1002/arch.21556
Laskowski M, Kato I (1980) Protein inhibitors of proteinases. Annu Rev Biochem 49:593–626. https://doi.org/10.1146/annurev.bi.49.070180.003113
Zhu L, Song L-S, Chang Y-Q et al (2006) Molecular cloning characterization and expression of a novel serine proteinase inhibitor gene in bay scallops (Argopecten irradians, Lamarck1819). Fish Shellfish Immunol 20:320–331. https://doi.org/10.1016/j.fsi.2005.05.009
Kato I (1987) Chicken ovomucoid: determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry 26:193–201. https://doi.org/10.1021/bi00375a027
Mägert HJ, Peter Kreutzmann P, Staendker L et al (2002) LEKTI: a multidomain serine proteinase inhibitor with pathophysiological relevance. Int J Biochem Cell Biol 34:573–576. https://doi.org/10.1016/S1357-2725(01)00179-0
Somprasong N, Rimphanitchayakit N, Tassanakajon A (2006) A five-domain Kazal-type serine proteinase inhibitor from black tiger shrimp Penaeus monodon and its inhibitory activities. Dev Comp Immunol 30:998–1008. https://doi.org/10.1016/j.dci.2006.01.004
Zhang D, Ma J, Jiang S (2014) Molecular characterization, expression and function analysis of a five-domain kazal-type serine proteinase inhibitor from pearl oyster Pinctada fucata. Fish Shellfish Immunol 37:115–121. https://doi.org/10.1016/j.fsi.2013.12.011
Soaresa TS, Buarquea BS, Queiroz DS et al (2015) A kazal-type inhibitor is modulated by Trypanosoma cruzi to control microbiota inside the anterior midgut of Rhodnius prolixus. Biochimie 112:41–48. https://doi.org/10.1016/j.biochi.2015.02.014
Sommerhoff CP, Söllner C, Piechottka GP et al (1994) A Kazal-type inhibitor of human mast cell tryptase: isolation from the medical leech Hirudo medicinalis, characterization, and sequence analysis. Biol Chem 375:685–694. https://doi.org/10.1515/bchm3.1994.375.10.685
Mende K, Petoukhova O, Koulitchkova V et al (1999) Dipetalogastin, a potent thrombin inhibitor from the blood-sucking insect Dipetalogaster maximus. Eur J Biochem 266:583–590. https://doi.org/10.1046/j.1432-1327.1999.00895.x
Chinese Pharmacopoeia Commission (2005) Pharmacopoeia of the People’s Republic of China. Chemical Industrial Press, Beijing
Chmelař J, Kotál J, Langhansová H et al (2017) Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction. Front Cell Infect Microbiol 7:216. https://doi.org/10.3389/fcimb.2017.00216
Calvo E, Mizurini DM, Sá-Nunes A et al (2011) Alboserpin, a factor Xa inhibitor from the mosquito vector of yellow fever, binds heparin and membrane phospholipids and exhibits antithrombotic activity. J Biol Chem 286:27998–28010. https://doi.org/10.1074/jbc.M111.247924
Lei L, Liu M, Shen J-J (2015) Cloning and expressing of Schistosoma japonicums serine protease inhibitor. Med Lab Sci Clinices 12:151–152
Cheng J-Z, Liu J, Zhai H et al (2017) Identification and expression of the Aedes aegypti serpin gene family. Chinese Journal of Parasitology and Parasitic Diseases 35:527–535. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZJSB201706003&DbName=CJFQ2017
Haycraft JB (1884) Ueber die Einwirkung eines Secretes des officinellen Blutegels auf die Gerinnbarkeit des Blutes. Archiv f. experiment. Pathol u Pharmakol 18:209–217. https://doi.org/10.1007/BF01833843
Davie E, Kulman J (2006) An overview of the structure and function of thrombin. Seminars in Thrombosis & Hemostasis 32:003–015. https://doi.org/10.1055/s-2006-939550
Müller C, Eickelmann C, Sponholz D et al (2021) Short tail stories: the hirudin-like factors HLF6 and HLF7 of the Asian medicinal leech, Hirudinaria manillensis. Parasitol Res 120:pages3761–3769. https://doi.org/10.1007/s00436-021-07316-3
Lukas P, Wolf R, Rauch BH et al (2019) Hirudins of the Asian medicinal leech, Hirudinaria manillensis: same same, but different. Parasitol Res 118:2223–2233. https://doi.org/10.1007/s00436-019-06365-z
Kama R, Chu X-H, Bao Z-X et al (2020) Enhanced anticoagulant activity of hirudin-i analogue co-expressed with arylsulfotransferase in periplasm of E. coli BL21(DE3). J Biotechnol 323:107–112. https://doi.org/10.1016/j.jbiotec.2020.08.003
Urata J, Shojo H, Kaneko Y (2003) Inhibition mechanisms of hematophagous invertebrate compounds acting on the host blood coagulation and platelet aggregation pathways. Biochimie 85:493–500. https://doi.org/10.1016/S0300-9084(03)00071-3
Christian M, Phil L, Sarah L et al (2019) Hirudin and Decorsins of the North American Medicinal Leech Macrobdella decora: Gene Structure Reveals Homology to Hirudins and Hirudin-Like Factors of Eurasian Medicinal Leeches. J Parasitol 105:423–431. https://doi.org/10.1645/18-117
Cheng R-M, Tang X-P, Long A-L et al (2019) Purification and characterization of a novel anti-coagulant from the leech Hirudinaria manillensis. Zool Res 40:205–210. https://doi.org/10.24272/j.issn.2095-8137.2019.037
Seymour JL, Henzel WJ, Nevins B et al (1990) Decorsin. A potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor from the leech Macrobdella decora. J Biol Chem 265. https://doi.org/10.1016/S0021-9258(19)38791-5. :10143-10143-10147
Ma J, Gao J-L (2021) Research advances in anticoagulant mechanisms of tick-derived protease inhibitors containing the Kunitz domain. Chin J Vector Biol & Control 32:111–114
Fink E, Rehm H, Gippner C et al (1986) The Primary Structure of Bdellin B-3 from the Leech Hirudo medicinalis. Bdellin B-3 is a Compact Proteinase Inhibitor of a “Non-classical” Kazal Type. It is present in the Leech in a High Molecular Mass Form. Biol Chem Hoppe-Seyler 367:1235–1242. https://doi.org/10.1515/bchm3.1986.367.2.1235
Locht A, Lamba D, Bauer M et al (1995) Two heads are better than one: crystal structure of the insect derived double domain kazal inhibitor rhodniin in complex with thrombin. EMBO J 14:5149–5157. https://doi.org/10.1002/j.1460-2075.1995.tb00199.x
Watanabe RM, Soares TS, Morais-Zani K et al (2010) A novel trypsin Kazal-type inhibitor from Aedes aegypti with thrombin coagulant inhibitory activity. Biochimie 92:933–939. https://doi.org/10.1016/j.biochi.2010.03.024
Zhang H-S, Qiao R-S, Gong H-Y et al (2017) Identification and anticoagulant activity of a novel Kunitz-type protein HA11 from the salivary gland of the tick Hyalomma asiaticum. Exp Appl Acarol 71:71–85. https://doi.org/10.1007/s10493-017-0106-1
Lemke S, Müller C, Hildebrandt JP (2016) Be ready at any time: postprandial synthesis of salivary proteins in salivary gland cells of the haematophagous leech Hirudo verbena. J Experimental Biology 219:1139–1145. https://doi.org/10.1242/jeb.135509
Wu M-J, Guo Q-S, Shi H-Z et al (2018) Cloning of Guamerin gene in Whitmania pigra and its spatio-temporal expression analysis after ingestion. China J Chin Materia Med 43:3605–3610. https://doi.org/10.19540/j.cnki.cjcmm.20180703.003
Cheng B-X, Liu F, Guo Q-S et al (2016) Identification and characterization of hirudin-HN, a new thrombin inhibitor, from the salivary glands of Hirudo nipponia. PeerJ 7:e7716. https://doi.org/10.7717/peerj.7716
Fan F-H, Kang X-X, Xie X et al (2021) Cloning and expression pattern of Hirudin-like factor gene by Hirudin-Like factor, Hirudo nipponica. J Guizhou Educ Univ 37:19–25. https://doi.org/10.13391/j.cnki.issn.1674-7798.2021.03.004
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The research invested, conceived, and designed was studied by Fei Liu, Boxing Cheng, and Yuxi Lu, Material preparation, data collection, and analysis were performed by Guiyan Shao, Qingqing Tian, Wen-Bo Li, and Suyan Wang. The first draft of the manuscript was written by Guiyan Shao, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Statement and declarations
This work was supported by the National Natural Science Foundation of China (Grant nos. 82073968), Guizhou Provincial Education Department Young Science and Technology Talents Development Project (KY [2021] 233), the Yancheng Institute of Technology Talent Introduction Project (XJR2021023), Major Program of Natural Science Research of Higher Education Institutions of Jiangsu Province (22KJA360011), Doctoral program of Guizhou Normal University (2020BS004) and youth foud of academy of agricultural sciences in Guizhou Province (2018(46)).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
All authors have seen the manuscript and approved to submit the manuscript.
Consent for publication
All authors consent to the publication of the manuscript.
Ethical approval
Ethical approval and informed consent are not required for the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, GY., Tian, QQ., Li, WB. et al. Cloning and functional identification of pmKPI cDNA in Poecilobdella manillensis. Mol Biol Rep 50, 299–308 (2023). https://doi.org/10.1007/s11033-022-07944-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07944-7