Abstract
Background
Breeding strategies to improve modern varieties having high yield, high nutritional value and resistance to biotic and abiotic stress, etc. is very important to make up for the food deficiencies. Molecular studies as a tool in breeding programs for the characterization of germplasm have been performed with several DNA marker systems.
Materials and methods
In the present study, the genetic diversity of 53 common bean landraces and 22 registered varieties from Turkey, and 12 genotypes from USDA was investigated using start codon targeted (SCoT) markers for the first time worldwide. The 8 primers having stronger and more polymorphic bands were used for PCR amplification.
Results
The mean polymorphic band of all primers was found as 13.13. The average of polymorphic information content and resolving power values was 0.34 and 7.55, respectively. Analysis of molecular variance (AMOVA) explored the existence of higher genetic diversity within populations accounting for 92% compared to among populations variations. According to cluster analysis (UPGMA) and genetic structure based on SCoT data, accessions were separated into Andean (PopA) and Mesoamerican PopB) gene pools. Moreover, accessions were mostly placed in the same groups/subgroups according to their geographical origin.
Conclusions
A high level of genetic diversity was observed between the investigated accessions in this work. The findings will help to plant breeders to characterize common bean accessions.




Similar content being viewed by others
References
Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Bean (Phaseolus spp.)-model food legumes. Plant Soil 252:55–128. https://doi.org/10.1023/A:1024146710611
Bitocchi E, Nannia L, Belluccia E et al (2012) Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. P Natl Acad Sci USA 109:788–796. https://. doi
Leitao ST, Dinis M, Veloso MM, Šatović Z, Vaz Patto MC (2017) Establishing the bases for introducing the unexplored Portuguese common bean germplasm into the breeding world. Front Plant Sci 8:1296. https://doi.org/10.3389/fpls.2017.01296
Gepts P, Osborn TC, Rashka K, Bliss FA (1986) Phaseolin protein variability in wild forms and landraces of the common bean (Phaseolus vulgaris): evidence for multiple centers of domestication. Econ Bot 40:451–468. https://doi.org/10.1007/BF02859659
Singh SP, Nodari R, Gepts P (1991) Genetic diversity in cultivated common bean: I. Allozymes. Crop Sci 31:19–23. https://doi.org/10.2135/cropsci1991.0011183X003100010004x
Schmutz J, McClean PE, Mamidi S et al (2014) A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet 46:707–713. https://doi.org/10.1038/ng.3008
Nadeem MA, Yeken MZ, Shahid MQ et al (2021) Common bean as a potential crop for future food security: an overview of past, current and future contributions in genomics, transcriptomics, transgenics and proteomics. Biotechnol Biotechnol Equip 35(1):758–786. https://doi.org/10.1080/13102818.2021.1920462
Bitocchi E, Rau D, Bellucci E, Rodriguez M, Murgia ML, Gioia T, Santo D, Nanni L, Attene G, Papa R (2017) Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front Plant Sci 8:722. https://doi.org/10.3389/fpls.2017.00722
Petry N, Boy E, Wirth JP, Hurrell RF (2015) Review: the potential of the common bean (Phaseolus vulgaris) as a vehicle for iron biofortification. Nutrients 7(2):1144–1173. https://doi.org/10.3390/nu7021144
Sperotto RA, Ricachenevsky FK (2017) Common bean Fe biofortification using model species’ lessons. Front Plant Sci 8:2187. https://doi.org/10.3389/fpls.2017.02187
FAOSTAT (2021) https://www.fao.org/faostat/en/#home Accessed 26 October 2021
Angioi SA, Rau D, Attene G et al (2010) Beans in Europe: origin and structure of the European landraces of Phaseolus vulgaris L. Theor Appl 121:829–843. https://doi.org/10.1007/s00122-010-1353-2
Şehirali S (1988) Yemeklik Dane Baklagiller. Ankara Universitesi Ziraat Fakultesi Class Book, no: 1089;
Nadeem MA, Habyarimana E, Çiftçi V et al (2018) Characterization of genetic diversity in Turkish common bean gene pool using phenotypic and whole-genome DArTseq-generated silicoDArT marker information. PLoS ONE 13(10):e0205363. https://doi.org/10.1371/journal.pone.0205363
Çelik A, Morca AF (2021) Development of colorimetric and real time loop-mediated isothermal amplification (cr-LAMP) assay for rapid detection of Wheat dwarf virus (WDV). Crop Prot 149:105786. https://doi.org/10.1016/j.cropro.2021.105786
Yeken MZ, Kantar F, Çancı H, Özer G, Çiftçi V (2018) Breeding of dry bean cultivars using Phaseolus vulgaris landraces in Turkey. Int J Agri Wild Sci 4(1):45–54. https://doi.org/10.24180/ijaws.408794
Yeken MZ, Özer G, Çelik A, Çíftçi V (2018) Identification of genes related to resistance for bean common mosaic virus and bean common mosaic necrosis virus in commercial common bean cultivars in Turkey. Turk J Agri Nat Sci 5:613–619. https://doi.org/10.30910/turkjans.471371
Palacioglu G, Bayraktar H, Ozer G (2020) Genetic variability of Colletotrichum lindemuthianum isolates from Turkey and resistance of Turkish bean cultivars. Span J Agric Res 18(3):e1005. https://doi.org/10.5424/sjar/2020183-16398
Saleh A, İmren M, Özer G, Yeken MZ, Çiftçi V, Dababat AA (2021) Host suitability of different common bean varieties in a growth room to the plant-parasitic nematodes Pratylenchus thornei and P. neglectus. Nematology, 1(aop):1–7. https://doi.org/10.1163/15685411-bja10105
Igwe DO, Afiukwa CA, Ubi BE, Ogbu KI, Ojuederie OB, Ude GN (2017) Assessment of genetic diversity in Vigna unguiculata L.(Walp) accessions using inter-simple sequence repeat (ISSR) and start codon targeted (SCoT) polymorphic markers. BMC Genet 18(1):1–13. https://doi.org/10.1186/s12863-017-0567-6
Aydın MF, Baloch FS (2018) Exploring the genetic diversity and population structure of Turkish common bean germplasm by the iPBS-retrotransposons markers. Legume Res LR –423:1–7. https://doi.org/10.18805/LR-423
Nemli S, Kianoosh T, Tanyolac MB (2015) Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) accessions through retrotransposon-based interprimer binding sites (iPBSs) markers. Turk J Agric For 39(6):940–948. https://doi.org/10.3906/tar-1505-59
Öztürk H, Dursun A, Hosseinpour A, Haliloğlu K (2020) Genetic diversity of pinto and fresh bean (Phaseolus vulgaris L.) germplasm collected from Erzincan province of Turkey by inter-primer binding site (iPBS) retrotransposon markers. Turk J Agric For 44(4):417–427. https://doi.org/10.3906/tar-2002-9
Kumar V, Sharma S, Kero S et al (2008) Assessment of genetic diversity in common bean (Phaseolus vulgaris L.) germplasm using amplified fragment length polymorphism (AFLP). Sci Hort 116(2):138–143. https://doi.org/10.1016/j.scienta.2007.12.001
Dagnew K, Haileselassie T, Feyissa T (2014) Genetic diversity study of common bean (Phaseolus vulgaris L.) germplasm from Ethiopia using inter simple sequence repeat (ISSR) markers. Afr J Biotechnol 13(36):3638–3649. http://. doi
Cabral PDS, de Souza LC, da Costa GF et al (2018) Investigation of the genetic diversity of common bean (Phaseolus vulgaris) cultivars using molecular markers. Genet Mol Res 17(4):1–11. https://doi.org/10.4238/gmr18106
Maras M, Sustar-Vozlic J, Javornik B, Meglic V (2008) The efficiency of AFLP and SSR markers in genetic diversity estimation and gene pool classification of common bean (Phaseolus vulgaris L.). Acta Agric Sloven 91:87–96. https://doi.org/10.2478/v10014-008-0009-2
Kwak M, Gepts P (2009) Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor Appl Genet 118:979–992. https://doi.org/10.1007/s00122-008-0955-4
Cabral PDS, Soares TCB, Lima ABP, de Miranda FD, Souza FB, Gonçalves LSA (2011) Genetic diversity in local and commercial dry bean (Phaseolus vulgaris) accessions based on microsatellite markers. Genet Mol Res 10:140–149. https://doi.org/10.4238/vol10-1gmr993
Wani AB, Bhat MA, Husaini AM, Sidiqi I (2017) Screening of important bean genotypes/collections for resistance against Common Bean Mosaic Virus using molecular markers. J Pharmacogn Phytochem 6(4):343–347. https://doi.org/10.6084/m9.figshare.12639305
Nemli S, Aşcioğul TK, Ateş D, Eşiyok D, Tanyolac MB (2017) Diversity and genetic analysis through DArTseq in common bean (Phaseolus vulgaris L.) germplasm from Turkey. Turk J Agric For 41(5):389–404. https://doi.org/10.3906/tar-1707-89
Valdisser PA, Pereira WJ, Almeida Filho JE et al (2017) In-depth genome characterization of a Brazilian common bean core collection using DArTseq high-density SNP genotyping. BMC Genomics 18(1):1–19. https://doi.org/10.1186/s12864-017-3805-4
Blair MW, Corte´s AJ, Penmetsa RV, Farmer A, Carrasquilla-Garcia N, Cook DR (2013) A high-throughput SNP marker system for parental polymorphism screening, and diversity analysis in common bean (Phaseolus vulgaris L.). Theor Appl Genet 126(2):535–548. https://doi.org/10.1007/s00122-012-1999-z
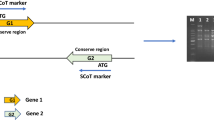
Collard BC, MackillD J (2009) Start codon targeted (SCoT) polymorphism, a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86. https://doi.org/10.1007/s11105-008-0060-5
Wang Y, Li S, Zhang X, Wang Y, Zhang C (2016) Isolation and analysis of differentially expressed genes during ovule abortion in the seedless grape. Sci Hortic 211:376–383. https://doi.org/10.1016/j.scienta.2016.09.014
Bhawna MZ, Arya L, Verma M (2017) Use of SCoT markers to assess the gene flowand population structure among two different population of bottle gourd. Plant Gene 9:80–86. https://doi.org/10.1016/j.plgene.2016.09.001
Gupta P, Mishra A, Lal RK, Dhawan SS (2021) DNA Fingerprinting and Genetic Relationships Similarities Among the Accessions/Species of Ocimum Using SCoT and ISSR Markers System. Mol Biotechnol 63:446–457. https://doi.org/10.1007/s12033-021-00316-9
Yılmaz A, Ciftci V (2021) Genetic Relationships and Diversity Analysis in Turkish Laurel (Laurus nobilis L.) Germplasm Using ISSR and SCoT Markers. https://doi.org/10.1007/s11033-021-06474-y. Molecular Biology Reports
Anonymous (2012) Project Report 109O163. TUBITAK. https://app.trdizin.gov.tr/proje/TVRRNU56SXk/dogu-anadolu-nun-guneyinde-yetistirilen-fasulye-gen-kaynaklarinin-toplanmasi-ve-degerlendirilmesi. Accessed 11 June 2021
Anonymous (2018) Project Report 115R042. TUBITAK. https://app.trdizin.gov.tr/proje/TVRrM01UYzM/ulusal-kuru-fasulye-gen-kaynaklarindan-yuksek-verimli-ve-antraknoz-colletotrichum-lindemuthianum-hastaligina-dayanikli-islah-materyallerinin-gelistirilmesi. Accessed 11 June 2021
Roldan-Ruiz I, Dendauw J, Vanbockstaele E et al (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed 6:125–134. https://doi.org/10.1023/A:1009680614564
Prevost A, Wilkinson MJ (1999) A new system of comparing PCR primers applied to ISSR finger printing of potato cultivars. Theor Appl Genet 98(1):107–112. https://doi.org/10.1007/ s001220051046
Peakall ROD, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6(1):288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Oksanen J, Blanchet FG, Friendly M et al (2020) Vegan: Community Ecology Package
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available at: http://www.R-project.org/
Pritchard JK, Stephens P, Donnelly P (2000) Inference of population structure using multi locus genotype data. Genetics 155(2):945–959
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14(8):2611–2620. https://doi.org/10.1111/j.1365-294x.2005.02553.x
Earl D, vonHoldt B (2011) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Guo X, Elston R (1999) Linkage information content of polymorphic genetic markers. Hum Hered 49(2):112–118. https://doi.org/10.1159/000022855
Etminan A, Pour-Aboughadareh A, Noori A, Ahmadi-Rad A, Shooshtari L, Mahdavian Z, Yousefiazar-Khanian M (2018) Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol Biotechnol Equip 32(3):610–617. https://doi.org/10.1080/13102818.2018.1447397
Hajibarat Z, Saidi A, Hajibarat Z, Talebi R (2015) Characterization of genetic diversity in chickpea using SSR markers, start codon targeted polymorphism (SCoT) and conserved DNA-derived polymorphism (CDDP). Physiol Mol Biol Pla 21(3):365–373. https://doi.org/10.1007/s12298-015-0306-2
Huded AKC, Jingade P, Bychappa M, Mishra MK (2020) Genetic diversity and population structure analysis of Coffee (Coffea canephora) germplasm collections in Indian Gene Bank employing SRAP and SCoT markers. Int J Fruit Sci 20(sup2):S757–S784. https://doi.org/10.1080/15538362.2020.1768618
Ghobadi G, Etminan A, Mehrabi AM, Shooshtari L (2021) Molecular diversity analysis in hexaploid wheat (Triticum aestivum L.) and two Aegilops species (Aegilops crassa and Aegilops cylindrica) using CBDP and SCoT markers. J Genet Eng Biotechnol 19(1):1–11. https://doi.org/10.1186/s43141-021-00157-8
Carović-Stanko K, Liber Z, Vidak M, Barešić A, Grdiša M, Lazarević B, Šatović Z (2017) Genetic diversity of Croatian common bean landraces. Front Plant Sci 8:604. https://doi.org/10.3389/fpls.2017.00604
Vidak M, Šatović Z, Liber Z et al (2021) Assessment of the Origin and Diversity of Croatian Common Bean Germplasm Using Phaseolin Type, SSR and SNP Markers and Morphological Traits. Plants 10(4):665. https://doi.org/10.3390/plants10040665
Barut M, Nadeem MA, Karaköy T et al (2020) DNA fingerprinting and genetic diversity analysis of world quinoa germplasm using iPBS-retrotransposon marker system. Turk J Agric Forest 44(5):479–491. https://doi.org/10.3906/tar-2001-10
Ana-Cruz MC, Helena ME, Yacenia MC (2017) Molecular characterization of Chenopodium quinoa Willd. using intersimple sequence repeat (ISSR) markers. Afr J Biotechnol 16(10):483–489. https://doi.org/10.5897/AJB2017.15925
Acknowledgements
We would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK; Project IDs: 115R042, 109O163 and 119R013), USDA-ARS Germplasm Resources Information Network (GRIN), Research Institutes of the Republic of Turkey Ministry of Agriculture and Forestry, and commercial companies for supplying plant materials used in this study. The authors are grateful to Bolu Abant Izzet Baysal University Scientific Research Projects Coordination Unit (2021.10.06.1495 and 2021.10.07.1518).
Author information
Authors and Affiliations
Contributions
Investigation, G.Ö., M.Z.Y., O.E.; Methodology, writing-review and editing G.Ö., M.Z.Y.; Statistical analysis, G.Ö., G.P., H.B.; Visualization, V.Ç., H.B.; Resources, V.Ç. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeken, M.Z., Emiralioğlu, O., Çiftçi, V. et al. Analysis of genetic diversity among common bean germplasm by start codon targeted (SCoT) markers. Mol Biol Rep 49, 3839–3847 (2022). https://doi.org/10.1007/s11033-022-07229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07229-z