Abstract
Background
Plant establishment, growth, development and productivity are adversely affected by abiotic stresses that are dominant characteristics of environmentally challenged/degraded habitats created in the Anthropocene. Crop breeding for climate resilience properties is need of the hour to sustain the crop productivity. We report on the characterization of Kappaphycus alvarezii (a red seaweed) Na+/H+ antiporter gene (KaNa+/H+) for enhanced salt and osmotic stress tolerance.
Methods
The KaNa+/H+ antiporter gene was cloned and over-expressed in tobacco under the control of CaMV35S promoter. Transgenic analysis was carried out to assess the stress tolerance ability of tobacco over-expressing KaNa+/H+ antiporter gene.
Results
Over-expression of KaNa+/H+ gene improved the seed germination and seed vigor index under stress. Transgenic plants grew better and exhibited delayed leaf senescence. Improved K+/Na+, carotenoid/total chlorophyll and relative water content; lower accumulation of reactive oxygen species (ROS), MDA and Na+; lower electrolyte leakage; better membrane stability index and accumulation of K+, photosynthetic pigment, starch, sugar, free amino acid, proline and polyphenol contents indicated better physiological health of the transgenic tobacco under stress. Transgenic tobacco exhibited higher photosynthesis, photosystem II efficiency, electron transfer rate, photochemical quenching and activity of water splitting complex. Compared with control tobacco, transgenic tobacco exhibited higher expression of stress-defence genes under stress and better recovery after long-term osmotic stress.
Conclusions
Lower Na+ cytotoxicity, lower accumulation of ROS and maintenance of the membrane integrity helped transgenic tobacco to maintain the physiological functioning under stress. Present results established K. alvarezii as a potential gene resource and the KaNa+/H+ antiporter gene as a potential candidate gene in molecular breeding of crops for development of the degraded land.









Similar content being viewed by others
References
FAO (2010) How to feed the world in 2050. https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf
Langridge P, Braun H, Hulke B, Ober E, Prasanna BM (2021) Breeding crops for climate resilience. Theor Appl Genet. https://doi.org/10.1007/s00122-021-03854-7
Tang X, Shao H, Jiang F, Sheteiwy MSA, Yang R, Wang H (2020) Molecular cloning and functional analyses of the salt responsive gene KVHSP70 from Kosteletzkya virginica. Land Degrad Dev 31:773–782. https://doi.org/10.1002/ldr.3503
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Review Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Zhao Q, Zhang H, Wang T, Chen S, Dai S (2013) Proteomics-based investigation of salt-responsive mechanisms in plant roots. J Proteom 82:230–253. https://doi.org/10.1016/j.jprot.2013.01.024
Yang L, Liu H, Fu SM, Ge HM, Tang RJ, YangY, Zhang HX (2017) Na+/H+ and K+/H+ antiporters AtNHX1 and AtNHX3 from Arabidopsis improve salt and drought tolerance in transgenic poplar. Biol Plant 61:641–650. https://doi.org/10.1007/s10535-017-0724-9
Kumari J, Udawat P, Dubey AK, Haque MI, Rathore MS, Jha B (2017) Overexpression of SbSI-1, a nuclear protein from Salicornia brachiata confers drought and salt stress tolerance and maintains photosynthetic efficiency in transgenic tobacco. Front Plant Sci 8:1215. https://doi.org/10.3389/fpls.2017.01215
Guo W, Li G, Wang N, Yang C, Zhao Y, Peng H, Chen S (2020) A Na+/H+ antiporter, K2-NhaD, improves salt and drought tolerance in cotton (Gossypium hirsutum L.). Plant Mol Biol 102:553–567. https://doi.org/10.1007/s11103-020-00969-1
Thudi M, Palakurthi R, Schnable JC, Chitikineni A, Dreisigacker S, Mace E, Varshney RK (2021) Genomic resources in plant breeding for sustainable agriculture. J Plant Physiol 257:153351. https://doi.org/10.1016/j.jplph.2020.153351
Rathore MS, Paliwal N, Anand KV, Agarwal PK (2015) Somatic embryogenesis and in vitro plantlet regeneration in Salicornia brachiata Roxb. Plant Cell Tissue Organ Cult (PCTOC) 120:355–360. https://doi.org/10.1007/s11240-014-0571-8
Kumari J, Rathore MS (2020) Na+ /K+—ATPase a primary membrane transporter: an overview and recent advances with special reference to algae. J Membr Biol 253:191–204. https://pubmed.ncbi.nlm.nih.gov/32430620/
Dubey AK, Khatri K, Jha B, Rathore MS (2021) The novel galactosyl transferase-like (SbGalT) gene from Salicornia brachiata maintains photosynthesis and enhances abiotic stress tolerance in transgenic tobacco. Gene 786:145597. https://doi.org/10.1016/j.gene.2021.145597
Yadav SK, Khatri K, Rathore MS, Jha B (2018) Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata delile enhanced photosynthesis and growth in tobacco. Mol Biol Rep 45:1745–1758. https://doi.org/10.1007/s11033-018-4318-1
Yadav SK, Khatri K, Rathore MS, Jha B (2020) Ectopic expression of a transmembrane protein KaCyt b6 from a red seaweed Kappaphycus alvarezii in transgenic tobacco augmented the photosynthesis and growth. DNA Cell Biol. https://doi.org/10.1089/dna.2020.5479
Guo R, Yang Z, Li F, Yan C, Zhong X, Liu Q, Zhao L (2015) Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol 15:1–13. https://doi.org/10.1186/s12870-015-0546-x
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499. https://doi.org/10.1146/annurev.arplant.51.1.463
Jha A, Joshi M, Yadav NS, Agarwal P, Jha B (2011) Cloning and characterization of the Salicornia brachiata Na+/H+ antiporter gene SbNHX1 and its expression by abiotic stress. Mol Biol Rep 38:1965–1973. https://doi.org/10.1007/s11033-010-0318-5
Haque MI, Rathore MS, Gupta H, Jha B (2017) Inorganic solutes contribute more than organic solutes to the osmotic adjustment in Salicornia brachiata (Roxb.) under natural saline conditions. Aquat Bot 142:78–86. https://doi.org/10.1016/j.aquabot.2017.07.004
Xu K, Hong P, Luo L, Xia T (2009) Overexpression of AtNHX1a, vacuolar Na+/H+ antiporter from Arabidopsis thalina, in Petunia hybrida enhances salt and drought tolerance. J Plant Biol 52:453–461. https://doi.org/10.1007/s12374-009-9058-2
Wang B, Zhai H, He S, Zhang H, Ren Z, Zhang D, Liu Q (2016) A vacuolar Na+/H+ antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci Hortic 201:153–166. https://doi.org/10.1016/j.scienta.2016.01.027
Zhang H, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768. https://doi.org/10.1038/90824
Lu W, Guo C, Li X, Duan W, Ma C, Zhao M, Gu J, Du X, Liu Z, Xiao K (2014) Overexpression of TaNHX3, a vacuolar Na+ /H+ antiporter gene in wheat, enhances salt stress tolerance in tobacco by improving related physiological processes. Plant Physiol Biochem 76:17–28. https://doi.org/10.1016/j.plaphy.2013.12.013
Joshi M, Jha A, Mishra A, Jha B (2013) Developing transgenic Jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS ONE. https://doi.org/10.1371/journal.pone.0071136
Yarra R, Kirti PB (2019) Expressing class I wheat NHX (TaNHX2) gene in eggplant improves plant performance under saline condition. Funct Integr Genomics 19:541–554. https://doi.org/10.1007/s10142-019-00656-5
Long L, Zhao JR, Guo DD, Ma XN, Xu FC, Yang WW, Gao W (2020) Identification of NHXs in Gossypium species and the positive role of GhNHX1 in salt tolerance. BMC Plant Biol 20:1–13. https://doi.org/10.1186/s12870-020-02345
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63:5727–5740. https://doi.org/10.1093/jxb/ers250
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142. https://doi.org/10.1105/tpc.111.095273
Rathore MS, Balar N, Jha B (2019) Population structure and developmental stage-associated ecophysiological responses of Salicornia brachiata. Ecol Res 34:644–658. https://doi.org/10.1111/1440-1703.12033
Tiwari V, Patel MK, Chaturvedi AK, Mishra A, Jha B (2019) Cloning and functional characterization of the Na+/H+ antiporter (NHX1) gene promoter from an extreme halophyte Salicornia brachiata. Gene 683:233–242. https://doi.org/10.1016/j.gene.2018.10.039
Zeng Y, Yang T (2002) RNA isolation from highly viscous samples rich in polyphenols and polysaccharides. Plant Mol Biol Rep 20:417–417. https://doi.org/10.1007/BF02772130
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262(47)
Walhout AJ, Temple GF, Brasch MA, Hartley JL, Lorson MA, Heuvel VDS, Vidal M (2000) GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol 328:575-IN7. https://doi.org/10.1016/S0076-6879(00)28419-X
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485. https://doi.org/10.1104/pp.77.2.483
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353. https://doi.org/10.1016/S0021-9258(19)85341-3
Sairam RK (1994) Effect of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress condition of two wheat varieties. Plant Growth Regul 14:173–181. https://doi.org/10.1007/BF00025220
Khatri K, Rathore MS (2019) Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 57:61–74. https://doi.org/10.32615/ps.2019.028
Hodges DM, DeLong M, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 20:604–611. https://doi.org/10.1007/s004250050524
He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288:2360–2363. https://doi.org/10.1126/science.288.5475.2360
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Chandler SF, Dodds JH (1983) The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep 2:205–208. https://doi.org/10.1007/BF00270105
Sugano N, Tanaka T, Yamamoto E, Nishi A (1975) Behaviour of phenylalanine ammonia-lyase in carrot cells in suspension cultures. Photochemistry 14:2435–2436. https://doi.org/10.1016/0031-9422(75)80359-1
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey MD, .Hickey LT (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nat Plants 4:23–29. https://doi.org/10.1038/s41477-017-0083-8
Hickey LT, Hafeez AN, Robinson H, Jackson SA, Leal-Bertioli SC, Tester M, Wulff BB (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37:744–754. https://doi.org/10.1038/s41587-019-0152-9
Uji T, Monma R, Mizuta H, Saga N (2012) Molecular characterization and expression analysis of two Na+/H+ antiporter genes in the marine red alga Porphyra yezoensis. Fish Sci 78:985–991. https://doi.org/10.1007/s12562-012-0520-6
Cai ZQ, Gao Q (2020) Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol 20:1–15. https://doi.org/10.1186/s12870-020-2279-8
Reguera M, Bassil E, Blumwald E (2014) Intracellular NHX-type cation/H+ antiporters in plants. Mol Plant 7:261–263. https://doi.org/10.1093/mp/sst091
Lakra N, Nutan KK, Das P, Anwar K, Singla-Pareek SL, Pareek A (2015) A nuclear-localized histone-gene binding protein from rice (OsHBP1b) functions in salinity and drought stress tolerance by maintaining chlorophyll content and improving the antioxidant machinery. J Plant Physiol 176:36–46. https://doi.org/10.1016/j.jplph.2014.11.005
Zhang X, Wollenweber B, Jiang D, Liu F, Zhao J (2008) Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J Exp Bot 59:839–848. https://doi.org/10.1093/jxb/erm364
Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Shabala S (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145:1714–1725. https://doi.org/10.1104/pp.107.110262
Chida H, Nakazawa A, Akazaki H, Hirano T, Suruga K, Ogawa M (2007) Expression of the algal cytochrome c6 gene in Arabidopsis enhances photosynthesis and growth. Plant Cell Physiol 48:948–957. https://doi.org/10.3389/fpls.2017.00582
Hasanuzzaman M, Davies NW, Shabala L, Zhou M, Brodribb TJ, Shabala S (2017) Residual transpiration as a component of salinity stress tolerance mechanism: a case study for barley. BMC Plant Biol 17:107. https://doi.org/10.1186/s12870-017-1054-y
Simkin AJ, McAusland L, Lawson T, Raines CA (2017) Overexpression of the RieskeFeS protein increases electron transport rates and biomass yield. Plant Physiol 175:134–145
Peixoto HP, Da Matta FM, Da Matta JC (2002) Responses of the photosynthetic apparatus to aluminum stress in two sorghum cultivars. J Plant Nutr 25:821–832. https://doi.org/10.1081/PLN-120002962
Ali MS, Kim K, Dhakal R, Choi D, Baek KH (2015) Accumulation of high contents of free amino acids in the leaves of Nicotiana benthamiana by the co-suppression of NbClpC1 and NbClpC2 genes. Plant Cell Rep 34:355–365. https://doi.org/10.1007/s00299-014-1714-4
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45:244–249. https://doi.org/10.1016/j.plaphy.2007.02.001
Singh VK, Mishra A, Haque I, Jha B (2016) A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci Rep 6:31686. https://doi.org/10.1038/srep31686
Acknowledgements
CSIR-CSMCRI Communication No. PRIS-60/2020. Authors acknowledge the CSIR, New Delhi for establishment of infrastructure facilities under different projects. JK acknowledge the kind help of Dr. A. Mishra during experimentation. JK acknowledge financial support of UGC, New Delhi as JRF/SRF and AcSIR, Ghaziabad for registration in Ph.D. program.
Author information
Authors and Affiliations
Contributions
MSR, JK—Conceptualized, conceived, and designed the experiments, JK, IH—Gene cloning and transgenic analysis, RKJ—Localization and RT-PCR, JK, IH, RKJ, MSR—Data analysis, JK, MSR—Drafting/editing and finalization of MS.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Ethical approval
The article does not contain any studies with animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
11033_2022_7213_MOESM1_ESM.pdf
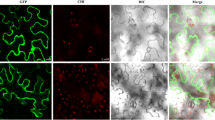
Supplementary file1 (PDF 2013 KB) Supplementary Figure S1 The nucleotide sequence of full-length KaNa+/H+ gene (a), deduced amino acids sequence KaNa+/H+ protein (b), trans-membrane domains in KaNa+/H+ antiporter protein (c), predicated secondary (d) and tertiary structure of KaNa+/H+ antiporter protein (e). Supplementary Figure S2 The in vivo localization construct for KaNa+/H+ protein (a), in vivo localization of KaNa+/H+ protein (b), PCR amplification of Uid A (c) and KaNa+/H+ (d) genes in transgenic tobacco. In gel pictures, the lane M denotes DNA ladder, PC- positive control, WT-wild type and lane Lx are transgenic tobacco lines. Supplementary Figure S3 Seed germination (a), germination index (b), seedling growth (c), root growth (d), fresh biomass (e), water contents (f), sodium contents (g) and potassium contents in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S4 Visual documentations of seedlings growth after 21 d in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S5 In vivo detection of accumulation of peroxide (a), superoxide (b) radicals in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S6 Tolerance index (a), and retention of total chlorophyll (b) chlorophyll a (c), chlorophyll b (d), carotenoid (e) contents and ratio of carotenoid and total chlorophyll contents (f) during senescence in leaves of WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S7 Leaf senescence assay in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure 8 Total chlorophyll (a), chlorophyll a (b), chlorophyll b (c), carotenoid (d) contents and the ratio of carotenoid and total chlorophyll (e) in WT, VC and KaNa+/H+ transgenic tobacco in WT, VC and KaNa+/H+ transgenic tobacco after 24 hours saline and osmotic stress. Supplementary Figure S9 Transpiration (a), WUE (b), vPDL (c), Ci/CA (d), ɸCO2 (e), FV/FM (f), FV’/FM’ (g), NPQ (h), F0/FM (i) and FV / F0 (j) in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S10 Starch (a), reducing sugar (b), stomatal conductance (c), intercellular CO2 concentration (d), qP (e), NPQ (f), accumulation of Na (g), accumulation of K (h) and ratio of K and Na (i) in WT, VC and KaNa+/H+ transgenic tobacco under saline and osmotic stress. Supplementary Figure S11 Morphological symptoms in WT, VC and KaNa+/H+ transgenic tobacco under 21 d long-term stress exposure and 7 d recovery in osmotic stress treated tobacco plants. Supplementary Figure S12 Principal component analysis for osmotic adjustment (a), physiological health (b), photosynthetic (c) and growth (d) responses in WT, VC and KaNa+/H+ transgenic tobacco under stress
Rights and permissions
About this article
Cite this article
Kumari, J., Haque, M.I., Jha, R.K. et al. The red seaweed Kappaphycus alvarezii antiporter gene (KaNa+/H+) confers abiotic stress tolerance in transgenic tobacco. Mol Biol Rep 49, 3729–3743 (2022). https://doi.org/10.1007/s11033-022-07213-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07213-7