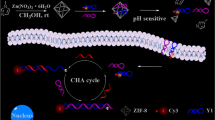
Abstract
Background
Accurately detecting and quantifying tumor-related microRNAs (miRNAs) in living cells is of great value for early cancer diagnosis. Herein, we present poly-adenine (polyA)-mediated spherical nucleic acid (SNA) nanoprobes for intracellular miRNA imaging in living cells.
Methods and results
polyA-mediated spherical nucleic acid (pASNA) nanoprobes consist of gold nanoparticles (AuNPs) anchored with fluorophore-labeled DNA molecules pre-hybridized with recognition sequences and polyA tails. The detection performance for miRNAs in vitro was studied to confirm the feasibility of pASNA nanoprobes for imaging live cell miRNAs. Before the pASNA nanoprobes were used for imaging intracellular miRNAs in MCF-7, HeLa, and LO2 cells, the stability and non-cytotoxicity were investigated using Dnase I and a standard colorimetric CCK8 assay. Flow cytometry, qRT-PCR analyses were conducted to confirm the different expression levels of miR-155 in live cells. Results showed that the pASNA nanoprobes had good detection sensitivity and specificity, excellent stability, and low toxicity. After incubating with pASNA nanoprobes, noticeable fluorescence signal enhancement could be clearly observed in MCF-7 and HeLa cells but not LO2 cells by confocal microscopy. Flow cytometry analysis and qRT-PCR indicated that MCF-7 and HeLa cells had higher miR-155 expression levels compared to LO2 cells.
Conclusions
The pASNA nanoprobes we developed had good sensitivity and specificity, excellent nuclease stability and low toxicity, thus representing a new approach to exquisitely reveal the distribution of endogenous miRNAs in live cells.




Similar content being viewed by others
References
He L, Thomson JM, Hemann MT et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833
Xing C, Chen Z, Lin Y et al (2021) Accelerated DNA tetrahedron-based molecular beacon for efficient microRNA imaging in living cells. Chem Commun (Camb) 57:3251–3254
Wong WK, Wong SHD, Bian L (2020) Long-term detection of oncogenic MicroRNA in living human cancer cells by Gold@ polydopamine-shell nanoprobe. ACS Biomater Sci Eng 6:3778–3783
Rossi JJ (2009) New hope for a microRNA therapy for liver cancer. Cell 137:990–992
Acunzo M, Romano G, Wernicke D, Croce CM (2015) MicroRNA and cancer–a brief overview. Adv Biol Regul 57:1–9
Pan W, Zhang T, Yang H, Diao W, Li N, Tang B (2013) Multiplexed detection and imaging of intracellular mRNAs using a four-color nanoprobe. Anal Chem 85:10581–10588
Li N, Chang C, Pan W, Tang B (2012) A multicolor nanoprobe for detection and imaging of tumor-related mRNAs in living cells. Angew Chem Int Ed Engl 51:7426–7430
Qiao G, Gao Y, Li N, Yu Z, Zhuo L, Tang B (2011) Simultaneous detection of intracellular tumor mRNA with bi-color imaging based on a gold nanoparticle/molecular beacon. Chemistry 17:11210–11215
Hou CH, Hou SM, Hsueh YS, Lin J, Wu HC, Lin FH (2009) The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 30:3956–3960
VanGuilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 44:619–626
Pall GS, Codony-Servat C, Byrne J, Ritchie L, Hamilton A (2007) Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res 35:60
Chen JJ (2007) Key aspects of analyzing microarray gene-expression data. Pharmacogenomics 8:473–482
Hu H, Zhou F, Wang B et al (2021) Autonomous operation of 3D DNA walkers in living cells for microRNA imaging. Nanoscale 13:1863–1868
Wang G, Guo Y, Liu Y, Zhou W, Wang G (2021) Algorithm-assisted detection and imaging of microRNAs in living cancer cells via the disassembly of plasmonic core-satellite probes coupled with strand displacement amplification. ACS Sens 6:958–966
Yu Y, Li L, Li G et al (2021) Intracellular enzyme-powered DNA circuit with a tunable amplifier for miRNA imaging. Chem Commun (Camb) 57:3753–3756
Liu Y, Li S, Zhang L, Zhao Q, Li N, Wu Y (2020) A sensitive and specific method for microRNA detection and in situ imaging based on a CRISPR–Cas9 modified catalytic hairpin assembly. RSC Adv 10:28037–28040
Dreaden E, Mackey M, Huang X, Kang B, El-Sayed M (2011) Beating cancer in multiple ways using nanogold. Chem Soc Rev 40:3391–3404
He Q, Zhang J, Chen F, Guo L, Zhu Z, Shi J (2010) An anti-ROS/hepatic fibrosis drug delivery system based on salvianolic acid B loaded mesoporous silica nanoparticles. Biomaterials 31:7785–7796
Qi H, Huang G, Han Y et al (2015) Engineering artificial machines from designable DNA materials for biomedical applications. Tissue Eng Part B 21:288–297
Pei H, Zuo X, Pan D, Shi J, Huang Q, Fan C (2013) Scaffolded biosensors with designed DNA nanostructures. NPG Asia Mater 5:e51–e51
Shi Y, Pan Y, Zhang H et al (2014) A dual-mode nanosensor based on carbon quantum dots and gold nanoparticles for discriminative detection of glutathione in human plasma. Biosens Bioelectron 56:39–45
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Wang L, Zhang H, Wang C et al (2019) Poly-adenine-mediated spherical nucleic acids for strand displacement-based DNA/RNA detection. Biosens Bioelectron 127:85–91
Li J, Huang J, Yang X et al (2018) Two-color-based nanoflares for multiplexed microRNAs imaging in live cells. Nanotheranostics 2:96–105
Wang C, Zhang H, Zeng D et al (2015) Elaborately designed diblock nanoprobes for simultaneous multicolor detection of microRNAs. Nanoscale 7:15822–15829
Jacobson DR, McIntosh DB, Stevens MJ, Rubinstein M, Saleh OA (2017) Single-stranded nucleic acid elasticity arises from internal electrostatic tension. Proc Natl Acad Sci USA 114:5095–5100
Peng H, Li XF, Zhang H, Le XC (2017) A microRNA-initiated DNAzyme motor operating in living cells. Nat Commun 8:14378
Zhu D, Zhao D, Huang J et al (2018) Poly-adenine-mediated fluorescent spherical nucleic acid probes for live-cell imaging of endogenous tumor-related mRNA. Nanomedicine 14:1797–1807
Zhang K, Hao L, Hurst SJ, Mirkin CA (2012) Antibody-linked spherical nucleic acids for cellular targeting. J Am Chem Soc 134:16488–16491
Zhang X, Servos MR, Liu J (2012) Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route. J Am Chem Soc 134:7266–7269
Zhang X, Liu B, Dave N, Servos MR, Liu J (2012) Instantaneous attachment of an ultrahigh density of nonthiolated DNA to gold nanoparticles and its applications. Langmuir 28:17053–17060
Blaya D, Aguilar-Bravo B, Hao F et al (2018) Expression of microRNA-155 in inflammatory cells modulates liver injury. Hepatology 68:691–706
Kono H, Nakamura M, Ohtsuka T et al (2013) High expression of microRNA-155 is associated with the aggressive malignant behavior of gallbladder carcinoma. Oncol Rep 30:17–24
Czyzyk-Krzeska MF, Zhang X (2014) MiR-155 at the heart of oncogenic pathways. Oncogene 33:677–678
Ezzat WM, Amr KS, Raouf HA et al (2016) Relationship Between Serum microRNA155 and Telomerase Expression in Hepatocellular Carcinoma. Arch Med Res 47:349–355
Yin Z, Guo H, Jiang K et al (2020) Morin decreases acrolein-induced cell injury in normal human hepatocyte cell line LO2. J Funct Foods 75:586
Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA (2007) Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett 7:3818–3821
Giljohann D, Seferos D, Daniel W, Massich M, Patel P, Mirkin C (2010) Gold nanoparticles for biology and medicine. Angew Chem 49:3280–3294
Sun JF, Zhang D, Gao CJ, Zhang YW, Dai QS (2019) Exosome-mediated MiR-155 transfer contributes to hepatocellular carcinoma cell proliferation by targeting PTEN. Med Sci Monit Basic Res 25:218–228
Liu X, Li Y, Li Z, Hou T (2021) miR-155 promotes proliferation and epithelial-mesenchymal transition of MCF-7 cells. Exp Ther Med 21:218
Fang H, Shuang D, Yi Z, Sheng H, Liu Y (2016) Up-regulated microRNA-155 expression is associated with poor prognosis in cervical cancer patients. Biomed Pharmacother 83:64–69
Santhekadur PK, Kumar DP (2020) RISC assembly and post-transcriptional gene regulation in Hepatocellular Carcinoma. Genes Dis 7:199–204
Sullivan RP, Fogel LA, Leong JW et al (2013) MicroRNA-155 tunes both the threshold and extent of NK cell activation via targeting of multiple signaling pathways. J Immunol 191:5904–5913
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (In vitro diagnostic technology and equipment), Science and Technology Service Network Initiative Program of the Chinese Academy of Sciences (KFJ-STS-QYZD-2021-08-002), Program of Shanghai Academic/Technology Research Leader (20XD1404600), Program of Shanghai Municipal Science and Technology Commission (20511107600).
Author information
Authors and Affiliations
Contributions
All authors contributed to the collection of reference, the contents of the article and the charts.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material includes DNA sequences, the gel electrophoresis image of ds-DNA, di-block polyA-DNA and reporter DNA, DLS results of AuNPs and pASNA nanoprobes, nuclease stability and cytotoxicity results and the optimized incubation time between tumor cells and pASNA nanoprobes. Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qian, Q., He, G., Wang, C. et al. Poly-adenine-mediated spherical nucleic acid probes for live cell fluorescence imaging of tumor-related microRNAs. Mol Biol Rep 49, 3705–3712 (2022). https://doi.org/10.1007/s11033-022-07210-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07210-w