Abstract
Background
In Arabidopsis, the genes SHOOT MERISTEMLESS (STM) and CLAVATA3 (CLV3) antagonistically regulate shoot meristem development. STM is essential for both development and maintenance of the meristem, as stm mutants fail to develop a shoot meristem. CLV3, on the other hand, negatively regulates meristem proliferation, and clv3 mutants possess an enlarged shoot meristem. Genetic interaction studies revealed that stm and clv3 dominantly suppress each other’s phenotypes. STM works in conjunction with its closely related homologue KNOTTED1-LIKE HOMEOBOX GENE 6 (KNAT6) to promote meristem development and organ separation, as stm knat6 double mutants fail to form shoot meristem and produce a fused cotyledon.
Results
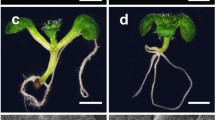
In this study, we show that clv3 fails to promote shoot meristem formation in stm-1 background if we also remove KNAT6. stm-1 knat6 clv3 triple mutants result in shoot meristem termination and produce fused cotyledons similar to stm knat6 double mutant. Notably, the stm-1 knat6 and stm-1 knat6 clv3 alleles lack tissue in the presumed region of SAM that is a novel phenotype reported in Arabidopsis mutants. stm-1 knat6 clv3 also showed reduced inflorescence size as compared to clv3 single or stm clv3 double mutants.
Conclusion
In contrast to previously published data, these data suggest that STM and KNAT6 are redundantly required for the vegetative SAM, but insufficient for the inflorescence meristem.




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author, Tie Liu, upon request.
Abbreviations
- SAM:
-
Shoot apical meristem
- NIC:
-
Nomarksi interference microscopy
- GR:
-
Glucocorticoid receptor
- Dex:
-
Dexamethasone (Dex)
References
Barton MK (2010) Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol
Walles B, Steeves TA, Sussex IM (1989) Patterns in plant development. Nord J Bot. https://doi.org/10.1111/j.1756-1051.1991.tb01820.x
Yadav RK, Tavakkoli M, Xie M, Girke T, Venugopala RG (2014) A high-resolution gene expression map of the arabidopsis shoot meristem stem cell niche. Development (Cambridge). https://doi.org/10.1242/dev.106104
Zhang WJ, Zhai LM, Yu HX, Peng J, Wang SS, Zhang XS, Su YH, Tang LP (2020) The BIG gene controls size of shoot apical meristems in Arabidopsis thaliana. Plant Cell Rep. https://doi.org/10.1007/s00299-020-02510-6
Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development
Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development
Lenhard M, Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development. https://doi.org/10.1242/dev.00525
Hake S (1996) Shootmeristemless ties the knot. Trends Plant Sci. https://doi.org/10.1016/s1360-1385(96)80036-7
Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development
Clark SE, Jacobsen SE., Levin JZ, Meyerowitz EM (1996) The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in arabidopsis. Development
Hay A, Tsiantis M (2010) KNOX genes: versatile regulators of plant development and diversity. Development
Jackson D, Veit B, Hake S (1994) Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. https://doi.org/10.1038/379066a0
Long J, Barton MK (2000) Initiation of axillary and floral meristems in Arabidopsis. Dev Biol. https://doi.org/10.1006/dbio.1999.9572
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. https://doi.org/10.1016/S0092-8674(03)00924-3
Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. https://doi.org/10.1105/tpc.12.4.507
Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. https://doi.org/10.1016/j.cub.2005.09.052
Cao X, Wang J, Xiong Y, Yang H, Yang M, Ye P, Bencivenga S, Sablowski R, Jiao Y (2020) A self-activation loop maintains meristematic cell fate for branching. Curr Biol. https://doi.org/10.1016/j.cub.2020.03.031
Schlegel J, Denay G, Pinto KG, Stahl Y, Schmid J, Blümke P, Simon R (2021) Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signaling pathways. bioRxiv. https://doi.org/10.1101/2021.06.14.448384
Su YH, Zhou C, Li YJ, Yu Y, Tang LP, Zhang WJ, Yao WJ, Huang R, Laux T, Zhang XS (2020) Integration of pluripotency pathways regulates stem cell maintenance in the arabidopsis shoot meristem. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2015248117
Brand U, Grünewald M, Hobe M, Simon R (2002) Regulation of CLV3 expression by two homeobox genes in Arabidopsis. Plant Physiol. https://doi.org/10.1104/pp.001867
Han H, Geng Y, Guo L, Yan A, Meyerowitz EM, Liu X, Zhou Y (2020) The overlapping and distinct roles of HAM family genes in arabidopsis shoot meristems. Front Plant Sci. https://doi.org/10.3389/fpls.2020.541968
Han H, Liu X, Zhou Y (2020b) Transcriptional circuits in control of shoot stem cell homeostasis. Curr Opin Plant Biol
Zhou Y, Yan A, Han H, Li T, Geng Y, Liu X, Meyerowitz EM (2018) Hairy meristem with wuschel confines clavata3 expression to the outer apical meristem layers. Science. https://doi.org/10.1126/science.aar8638
Zhang L, Gennaro De Lin DG, Chai J, Shpak ED (2021) ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem. Development (Cambridge). https://doi.org/10.1242/dev.189753
Zhang TQ, Chen Y, Wang JW (2021) A single-cell analysis of the Arabidopsis vegetative shoot apex. Dev Cell. https://doi.org/10.1016/j.devcel.2021.02.021
De Marchis F, Colanero S, Klein EM, Mainieri D, Prota VM, Bellucci M, Pagliuca G, Zironi E, Gazzotti T, Vitale A et al (2018) Expression of CLAVATA3 fusions indicates rapid intracellular processing and a role of ERAD. Plant Sci. https://doi.org/10.1016/j.plantsci.2018.03.020
Somssich M, Je BIl, Simon R, Jackson D (2016) CLAVATA-WUSCHEL signaling in the shoot meristem. Development (Cambridge)
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell. https://doi.org/10.1105/tpc.9.6.841
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development
Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MACJ, De Vries SC (2003) The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell. https://doi.org/10.1105/tpc.012203
Takada S, Hibara KI, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development
Boscá S, Knauer S, Laux T (2011) Embryonic development in arabidopsis thaliana: From the zygote division to the shoot meristem. Front Plant Sci
Kwon CS, Hibara KI, Pfluger J, Bezhani S, Metha H, Aida M, Tasaka M, Wagner D (2006) A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development. https://doi.org/10.1242/dev.02508
Spinelli SV, Martin AP, Viola IL, Gonzalez DH, Palatnik JF (2011) A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol. https://doi.org/10.1104/pp.111.177709
Balkunde R, Kitagawa M, Xu XM, Wang J, Jackson D (2017) SHOOT MERISTEMLESS trafficking controls axillary meristem formation, meristem size and organ boundaries in Arabidopsis. Plant J. https://doi.org/10.1111/tpj.13504
Turner S, Sieburth LE (2003) Vascular patterning. The arabidopsis book. https://doi.org/10.1199/tab.0073
Hake S, Smith HMS, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of Knox genes in plant development. Ann Rev Cell Dev Biol
Scofield S, Murray JAH (2006) The evolving concept of the meristem. Plant Mol Biol
Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD (2002) KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. https://doi.org/10.1105/tpc.010391
Smith HMS, Hake S (2003) The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell. https://doi.org/10.1105/tpc.012856
Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, Martienssen R, Selvaraj G, Datla R (2002) The homeobox gene brevipedicellus is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.072626099
Byrne ME, Simorowski J, Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals know gene redundancy in Arabidopsis. Development
Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, Pautot V (2006) KNAT6: an arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell. https://doi.org/10.1105/tpc.106.041988
Aida M, Tsubakimoto Y, Shimizu S, Ogisu H, Kamiya M, Iwamoto R, Takeda S, Karim MR, Mizutani M, Lenhard M et al (2020) Establishment of the embryonic shoot meristem involves activation of two classes of genes with opposing functions for meristem activities. Int J Mol Sci. https://doi.org/10.3390/ijms21165864
Hake S (2019) Identification of cup-shaped cotyledon: new ways to think about organ initiation. Plant Cell
Scofield S, Murison A, Jones A, Fozard J, Aida M, Band LR, Bennett M, Murray JAH (2018) Coordination of meristem and boundary functions by transcription factors in the SHOOT MERISTEMLESS regulatory network. Development (Cambridge). https://doi.org/10.1242/dev.157081
Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. https://doi.org/10.1101/gad.905201
Guo X, Lu W, Ma Y, Qin Q, Hou S (2013) The BIG gene is required for auxin-mediated organ growth in Arabidopsis. Planta. https://doi.org/10.1007/s00425-012-1834-4
Tian C, Wang Y, Yu H, He J, Wang J, Shi B, Du Q, Provart NJ, Meyerowitz EM, Jiao Y (2019) A gene expression map of shoot domains reveals regulatory mechanisms. Nat Commun. https://doi.org/10.1038/s41467-018-08083-z
Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM (2015) Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development (Cambridge). https://doi.org/10.1242/dev.119677
Perales M, Reddy GV (2012) Stem cell maintenance in shoot apical meristems. Curr Opin Plant Biol
Laux T, Mayer KFX, Berger J, Jürgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development
Heath TL (1897) The works of Archimedes. Dover Publications, Mineola, NY, p 115
Acknowledgements
We thank Dr. Kathryn M. Barton and the Carnegie Institute for Science for supporting the work at Carnegie Institution of Science, Stanford, CA. We also thank Dr. Véronique Pautot for providing knat6-1 seeds. This work is partially supported by University of Florida Seed Grant and startup fund.
Funding
This research was supported by at Carnegie Institution of Science, Stanford, CA via funding to Dr. Kathryn M. Barton. This work is partially supported by University of Florida Seed Grant and startup fund.
Author information
Authors and Affiliations
Contributions
NS conceptualization, investigation, data curation, formal analysis, methodology, validation, visualization, writing—original draft, writing—review & editing; TL Writing—review and editing; KMB conceptualization, funding acquisition, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nidhi, S., Preciado, J. & Tie, L. Knox homologs shoot meristemless (STM) and KNAT6 are epistatic to CLAVATA3 (CLV3) during shoot meristem development in Arabidopsis thaliana. Mol Biol Rep 48, 6291–6302 (2021). https://doi.org/10.1007/s11033-021-06622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06622-4