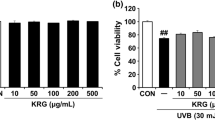
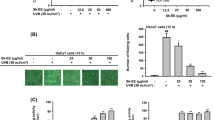
Abstract
Matrix metalloproteinase 1 (MMP-1) initiates the breakdown of matrix networks by cleaving fibrillar collagen during the pathophysiological progression of skin aging. Ageratum houstonianum ethanol extract (AHE) has been used as a traditional herbal medicine to treat external wounds and skin diseases. However, the mechanism of action underlying A. houstonianum-mediated modulation of skin aging has not been investigated. In this study, we evaluated the effect of AHE on MMP-1 expression in HaCaT keratinocytes. Gene expression was analyzed by Reverse transcription-PCR (RT-PCR), Quantitative real-time PCR (Q-PCR), gene promoter-reporter assay, and immunoblotting. We found that AHE abrogated TNFα-induced MMP1 expression at the transcriptional level via the suppression of ERK1/2 mitogen-activated protein kinase (MAPK)-mediated Early Growth Response 1 (EGR1) expression. We also demonstrated that β-caryophyllene, a cannabinoid receptor 2 (CB2) agonist, is a functional component of the AHE that inhibits TNFα-induced EGR-1 and MMP1 expression. AHE exerts inhibitory activity on TNFα-induced MMP1 expression at the transcription level through EGR-1 downregulation in keratinocytes. β-Caryophyllene is a bioactive ingredient of AHE that is responsible for the inhibition of TNFα-induced EGR1 expression. β-Caryophyllene can be used as a potential agent to prevent inflammation-induced skin aging.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AHE:
-
Ageratum houstonianum ethanol extract
- EGR1:
-
Early Growth Response 1
- JNK:
-
c-Jun NH2-terminal kinase
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- IL:
-
interleukin
- Q-PCR:
-
quantitative real-time PCR
- MMP-1:
-
matrix metalloproteinase 1
- MAPK:
-
mitogen-activated protein kinase
- RT-PCR:
-
reverse transcription-PCR
- TNFα:
-
tumor necrosis factor α
References
Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ (2002) Mechanisms of photoaging and chronological skin aging. Arch Dermatol 138(11):1462–1470. https://doi.org/10.1001/archderm.138.11.1462
Kahari VM, Saarialho-Kere U (1997) Matrix metalloproteinases in skin. Exp Dermatol 6(5):199–213
Brenneisen P, Sies H, Scharffetter-Kochanek K (2002) Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci 973:31–43. https://doi.org/10.1111/j.1749-6632.2002.tb04602.x
Westermarck J, Li S, Jaakkola P, Kallunki T, Grenman R, Kahari VM (2000) Activation of fibroblast collagenase-1 expression by tumor cells of squamous cell carcinomas is mediated by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase-2. Cancer Res 60(24):7156–7162
Han Z, Boyle DL, Aupperle KR, Bennett B, Manning AM, Firestein GS (1999) Jun N-terminal kinase in rheumatoid arthritis. J Pharmacol Exp Ther 291(1):124–130
Johansson N, Ala-aho R, Uitto V, Grenman R, Fusenig NE, Lopez-Otin C, Kahari VM (2000) Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci 113(Pt 2):227–235
eFlora Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA, USA. http://www.efloras.org. Accessed 2016.01.10
Zeeshan M, Rizvi SM, Khan MS, Kumar A (2012) Isolation, partial purification and evaluation of bioactive compounds from leaves of Ageratum houstonianum. EXCLI J 11:78–88
Verma A, Rizvi SM, Shaikh S, Ansari MA, Shakil S, Ghazal F, Siddiqui MH, Haneef M, Rehman A (2014) Compounds isolated from Ageratum houstonianum inhibit the activity of matrix metalloproteinases (MMP-2 and MMP-9): an oncoinformatics study. Pharmacogn Mag 10(37):18–26 doi: 10.4103/0973-1296.126653 PM-10-18 [pii]
Wiedenfeld H, Andrade-Cetto A (2001) Pyrrolizidine alkaloids from Ageratum houstonianum mill. Phytochemistry 57(8):1269–1271 doi: S0031-9422(01)00192-3 [pii]
Kumar N (2014) Biological potential of a weed Ageratum houstonianum mill:a review. Indo Am J Pharm Res 4(6):2683–2689
Kurade NP, Jaitak V, Kaul VK, Sharma OP (2010) Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm Biol 48(5):539–544. https://doi.org/10.3109/13880200903193336
Pandey DK, Chandra H, Tripathi NN, Dixit SN (1983) Mycotoxicity in leaves of some higher plants with special reference to that of Ageratum houstonianum mill. Mykosen 26(11):565–573
Menut C, Lamaty G, Zollo PHA, Kuiate JR, Bessiere JM (1993) Aromatic plants of tropical Central Africa. Part X. Chemical composition of the essential oils of Ageratum houstonianum Mill. and Ageratum conyzoides L. from Cameroon. Flavour Fragrance J 8(1):1–4
Singh B, Singh., J. (2014) Ethnobotanical uses of some plants from Central Haryana, India. Phytodiversity 1 (1&2):7–24
Shin SY, Lee DH, Gil HN, Kim BS, Choe JS, Kim JB, Lee YH, Lim Y (2017) Agerarin, identified from Ageratum houstonianum, stimulates circadian CLOCK-mediated aquaporin-3 gene expression in HaCaT keratinocytes. Sci Rep 7(1):11175. https://doi.org/10.1038/s41598-017-11642-x
Xu W, Jia S, Xie P, Zhong A, Galiano RD, Mustoe TA, Hong SJ (2014) The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J Invest Dermatol 134(4):1044–1055 quiz e1015. doi: S0022-202X(15)36711-7 [pii] 10.1038/jid.2013.425
Shin SY, Kim JH, Baker A, Lim Y, Lee YH (2010) Transcription factor Egr-1 is essential for maximal matrix metalloproteinase-9 transcription by tumor necrosis factor alpha. Mol Cancer Res 8(4):507–519 doi: 1541-7786.MCR-09-0454 [pii] 10.1158/1541-7786.MCR-09-0454
Yeo H, Lee JY, Kim J, Ahn SS, Jeong JY, Choi JH, Lee YH, Shin SY (2020) Transcription factor EGR-1 transactivates the MMP1 gene promoter in response to TNFalpha in HaCaT keratinocytes. BMB Rep 53(6):323–328
Sun Y, Wenger L, Brinckerhoff CE, Misra RR, Cheung HS (2002) Basic calcium phosphate crystals induce matrix metalloproteinase-1 through the Ras/mitogen-activated protein kinase/c-Fos/AP-1/metalloproteinase 1 pathway. Involvement of transcription factor binding sites AP-1 and PEA-3. J Biol Chem 277(2):1544–1552. https://doi.org/10.1074/jbc.M100567200
Westermarck J, Seth A, Kahari VM (1997) Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene 14(22):2651–2660. https://doi.org/10.1038/sj.onc.1201111
Grimbacher B, Aicher WK, Peter HH, Eibel H (1998) TNF-alpha induces the transcription factor Egr-1, pro-inflammatory cytokines and cell proliferation in human skin fibroblasts and synovial lining cells. Rheumatol Int 17(5):185–192. https://doi.org/10.1007/s002960050032
Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB (2009) Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem 284(4):2275–2284
Mishra JP, Mishra S, Gee K, Kumar A (2005) Differential involvement of calmodulin-dependent protein kinase II-activated AP-1 and c-Jun N-terminal kinase-activated EGR-1 signaling pathways in tumor necrosis factor-alpha and lipopolysaccharide-induced CD44 expression in human monocytic cells. J Biol Chem 280(29):26825–26837
Xiao Nan Lu XCL, Liu QZ, Liu ZL (2014, 2014) Isolation of insecticidal constituents from the essential oil of Ageratum houstonianum mill. Against Liposcelis bostrychophila Badonnel. J Chem. https://doi.org/10.1155/2014/645687
Sharma C, Al Kaabi JM, Nurulain SM, Goyal SN, Kamal MA, Ojha S (2016) Polypharmacological properties and therapeutic potential of beta-Caryophyllene: a dietary Phytocannabinoid of pharmaceutical promise. Curr Pharm Des 22(21):3237–3264. https://doi.org/10.2174/1381612822666160311115226
Basu S, Ray A, Dittel BN (2011) Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses. J Immunol 187(11):5720–5732. https://doi.org/10.4049/jimmunol.1102195
Adhikary S, Kocieda VP, Yen JH, Tuma RF, Ganea D (2012) Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood 120(18):3741–3749. https://doi.org/10.1182/blood-2012-06-435362
Gui H, Liu X, Wang ZW, He DY, Su DF, Dai SM (2014) Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 53(5):802–809. https://doi.org/10.1093/rheumatology/ket447
Yoshizaki N, Fujii T, Hashizume R, Masaki H (2016) A polymethoxyflavone mixture, extracted from orange peels, suppresses the UVB-induced expression of MMP-1. Exp Dermatol 25(Suppl 3):52–56. https://doi.org/10.1111/exd.13087
Bastiaansen-Jenniskens YM, Siawash M, van de Lest CH, Verhaar JA, Kloppenburg M, Zuurmond AM, Stojanovic-Susulic V, Van Osch GJ, Clockaerts S (2013) Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: a preliminary study. Cartilage 4(4):321–328. https://doi.org/10.1177/1947603513494401
Koyama S, Purk A, Kaur M, Soini HA, Novotny MV, Davis K, Kao CC, Matsunami H, Mescher A (2019) Beta-caryophyllene enhances wound healing through multiple routes. PLoS One 14(12):e0216104. https://doi.org/10.1371/journal.pone.0216104
Lucca LG, de Matos SP, Borille BT, Teixeira HF, Veiga VF Jr, Limberger RP, Koester LS (2015) Determination of beta-caryophyllene skin permeation/retention from crude copaiba oil (Copaifera multijuga Hayne) and respective oil-based nanoemulsion using a novel HS-GC/MS method. J Pharm Biomed Anal 104:144–148. https://doi.org/10.1016/j.jpba.2014.11.013
Acknowledgements
The paper was supported by the KU Research Professor Program of Konkuk University.
Funding
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2020R1A2C1005845).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Soon Young Shin, Dongsoo Koh and Yoongho Lim. The first draft of the manuscript was written by Soon Young Shin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Consent to participate
All authors have seen the manuscript and approved to submit the manuscript.
Consent for publication
All authors consent to the publication of the manuscript.
Ethical approval
Ethical approval and informed consent not required for the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shin, S.Y., Koh, D., Lim, Y. et al. Inhibition of EGR-1-dependent MMP1 transcription by ethanol extract of Ageratum houstonianum in HaCaT keratinocytes. Mol Biol Rep 48, 1–11 (2021). https://doi.org/10.1007/s11033-020-06091-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06091-1