Abstract
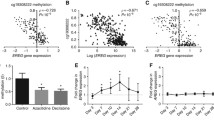
The G-protein coupled estrogen receptor (GPER), a proposed tumor suppressor, relays short-term non-genomic responses in target cells and tissues. It frequently undergoes down-modulation in primary tumors of the breast, ovary, and endometrium. Liu and co-workers recently reported loss of GPER expression in colorectal cancer and attributed it to DNA methylation-dependent silencing. We hypothesized that GPER expression is inversely correlated with methylation in the upstream CpG island (upCpGi) in the GPER locus. Methylation in the upCpGi was analysed by bisulfite sequencing and correlated with GPER expression in a panel of colon cancer cell lines. Eight downstream CpGs of the upCpGi was differentially methylated across the cell lines. Methylation in this differentially methylated region (DMR) correlated inversely with GPER expression. Two cell lines, namely SW620 and COLO-320DM, were compared in terms of their viability in response to varying concentrations of G1, a GPER specific agonist. SW-620 cells, which had the least methylated DMR and the highest level of GPER expression, showed significant loss of viability with 1 µM G1. COLO-320DM, which had the most methylated DMR and the lowest level of GPER expression, did not show a significant response to 1 µM G1. At 5 µM G1, SW620 cells showed a greater reduction in viability than COLO-320DM cells. DNA methylation in the DMR is inversely correlated with GPER expression. DNA methylation-dependent silencing of GPER may be, at least in part, the underlying reason behind the loss of estrogen’s oncoprotective effect via GPER in the colon.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Simon MS, Chlebowski RT, Wactawski-Wende J et al (2012) Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol 30:3983–3990. https://doi.org/10.1200/JCO.2012.42.7732
Lin KJ, Cheung WY, Lai JY-C, Giovannucci EL (2012) The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J cancer 130:419–430. https://doi.org/10.1002/ijc.26026
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333. https://doi.org/10.1001/jama.288.3.321
Quaresma M, Coleman MP, Rachet B (2015) 40-Year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet 385:1206–1218. https://doi.org/10.1016/S0140-6736(14)61396-9
Nilsson S, Mäkelä S, Treuter E et al (2001) Mechanisms of estrogen action. Physiol Rev 81:1535–1565. https://doi.org/10.1152/physrev.2001.81.4.1535
Hammes SR, Levin ER (2007) Extranuclear steroid receptors: nature and actions. Endocrinol Rev 28:726–741. https://doi.org/10.1210/er.2007-0022
Hammes SR, Levin ER (2011) Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology 152:4489–4495. https://doi.org/10.1210/en.2011-1470
Lange CA, Gioeli D, Hammes SR, Marker PC (2007) Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol 69:171–199. https://doi.org/10.1146/annurev.physiol.69.031905.160319
Losel RM, Falkenstein E, Feuring M et al (2003) Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 83:965–1016. https://doi.org/10.1152/physrev.00003.2003
Chaudhri RA, Schwartz N, Elbaradie K et al (2014) Role of ERα36 in membrane-associated signaling by estrogen. Steroids 81:74–80. https://doi.org/10.1016/j.steroids.2013.10.020
Sołtysik K, Czekaj P (2015) ERα36–another piece of the estrogen puzzle. Eur J Cell Biol 94:611–625. https://doi.org/10.1016/j.ejcb.2015.10.001
Xu S, Yu S, Dong D, Lee LTO (2019) G protein-coupled estrogen receptor: a potential therapeutic target in cancer. Front Endocrinol (Lausanne) 10:725. https://doi.org/10.3389/fendo.2019.00725
Kennelly R, Kavanagh DO, Hogan AM, Winter DC (2008) Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol 9:385–391. https://doi.org/10.1016/S1470-2045(08)70100-1
Papaxoinis K, Triantafyllou K, Sasco AJ et al (2010) Subsite-specific differences of estrogen receptor beta expression in the normal colonic epithelium: implications for carcinogenesis and colorectal cancer epidemiology. Eur J Gastroenterol Hepatol 22:614–619. https://doi.org/10.1097/MEG.0b013e328335ef50
Konstantinopoulos PA, Kominea A, Vandoros G et al (2003) Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer 39:1251–1258. https://doi.org/10.1016/s0959-8049(03)00239-9
Jassam N, Bell SM, Speirs V, Quirke P (2005) Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep 14:17–21
Elbanna HG, Ebrahim MA, Abbas AM et al (2012) Potential value of estrogen receptor beta expression in colorectal carcinoma: interaction with apoptotic index. J Gastrointest Cancer 43:56–62. https://doi.org/10.1007/s12029-010-9214-4
Rudolph A, Toth C, Hoffmeister M et al (2012) Expression of oestrogen receptor β and prognosis of colorectal cancer. Br J Cancer 107:831–839. https://doi.org/10.1038/bjc.2012.323
Saleiro D, Murillo G, Benya RV et al (2012) Estrogen receptor-β protects against colitis-associated neoplasia in mice. Int J cancer 131:2553–2561. https://doi.org/10.1002/ijc.27578
Williams C, DiLeo A, Niv Y, Gustafsson J-Å (2016) Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett 372:48–56. https://doi.org/10.1016/j.canlet.2015.12.009
Prossnitz ER, Barton M (2009) Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat 89:89–97. https://doi.org/10.1016/j.prostaglandins.2009.05.001
Maggiolini M, Picard D (2010) The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 204:105–114. https://doi.org/10.1677/JOE-09-0242
Smith HO, Leslie KK, Singh M et al (2007) GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol 196:386.e1-386.e11. https://doi.org/10.1016/j.ajog.2007.01.004
Smith HO, Arias-Pulido H, Kuo DY et al (2009) GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol 114:465–471. https://doi.org/10.1016/j.ygyno.2009.05.015
Prossnitz ER, Barton M (2014) Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol 389:71–83. https://doi.org/10.1016/j.mce.2014.02.002
Gaudet HM, Cheng SB, Christensen EM, Filardo EJ (2015) The G-protein coupled estrogen receptor, GPER: the inside and inside-out story. Mol Cell Endocrinol 418 Pt 3:207–219. https://doi.org/10.1016/j.mce.2015.07.016
Barton M (2016) Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids 111:37–45. https://doi.org/10.1016/j.steroids.2016.02.016
Jacenik D, Beswick EJ, Krajewska WM, Prossnitz ER (2019) G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J Gastroenterol 25:4092–4104. https://doi.org/10.3748/wjg.v25.i30.4092
Liu Q, Chen Z, Jiang G et al (2017) Epigenetic down regulation of G protein-coupled estrogen receptor (GPER) functions as a tumor suppressor in colorectal cancer. Mol Cancer 16:87. https://doi.org/10.1186/s12943-017-0654-3
Bustos V, Nolan ÁM, Nijhuis A et al (2017) GPER mediates differential effects of estrogen on colon cancer cell proliferation and migration under normoxic and hypoxic conditions. Oncotarget 8:84258–84275. https://doi.org/10.18632/oncotarget.20653
Weißenborn C, Ignatov T, Poehlmann A et al (2014) GPER functions as a tumor suppressor in MCF-7 and SK-BR-3 breast cancer cells. J Cancer Res Clin Oncol 140:663–671. https://doi.org/10.1007/s00432-014-1598-2
Weissenborn C, Ignatov T, Nass N et al (2017) GPER promoter methylation controls GPER expression in breast cancer patients. Cancer Invest 35:100–107. https://doi.org/10.1080/07357907.2016.1271886
Manjegowda MC, Gupta PS, Limaye AM (2017) Hyper-methylation of the upstream CpG island shore is a likely mechanism of GPER1 silencing in breast cancer cells. Gene 614:65–73. https://doi.org/10.1016/j.gene.2017.03.006
Manjegowda MC, Gupta PS, Limaye AM (2016) Validation data of a rabbit antiserum and affinity purified polyclonal antibody against the N-terminus of human GPR30. Data Br 7:1015–1020. https://doi.org/10.1016/j.dib.2016.03.054
LOWRY OH, ROSEBROUGH NJ, RANDALL FARRAL RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manjegowda MC, Deb G, Limaye AM (2014) Epigallocatechin gallate induces the steady state mRNA levels of pS2 and PR genes in MCF-7 breast cancer cell. Indian J Exp Biol 52:312–316
John Mary DJS, Sikarwar G, Kumar A, Limaye AM (2020) Interplay of ERα binding and DNA methylation in the intron-2 determines the expression and estrogen regulation of cystatin A in breast cancer cells. Mol Cell Endocrinol 504:110701. https://doi.org/10.1016/j.mce.2020.110701
Santolla MF, Lappano R, De Marco P et al (2012) G protein-coupled estrogen receptor mediates the up-regulation of fatty acid synthase induced by 17β-estradiol in cancer cells and cancer-associated fibroblasts. J Biol Chem 287:43234–43245. https://doi.org/10.1074/jbc.M112.417303
Gilligan LC, Gondal A, Tang V et al (2017) Estrone sulfate transport and steroid sulfatase activity in colorectal cancer: implications for hormone replacement therapy. Front Pharmacol 8:103. https://doi.org/10.3389/fphar.2017.00103
Gilligan LC, Rahman HP, Hewitt A-M et al (2017) Estrogen activation by steroid sulfatase increases colorectal cancer proliferation via GPER. J Clin Endocrinol Metab 102:4435–4447. https://doi.org/10.1210/jc.2016-3716
Weißenborn C, Ignatov T, Ochel H-J et al (2014) GPER functions as a tumor suppressor in triple-negative breast cancer cells. J Cancer Res Clin Oncol 140:713–723. https://doi.org/10.1007/s00432-014-1620-8
Lv Q-Y, Xie B-Y, Yang B-Y et al (2017) Increased TET1 expression in inflammatory microenvironment of hyperinsulinemia enhances the response of endometrial cancer to estrogen by epigenetic modulation of GPER. J Cancer 8:894–902. https://doi.org/10.7150/jca.17064
Acknowledgements
The work was supported by financial assistance from the Indian Council of Medical Research, Govt. of India (Sanction letter No. 2019 − 0687/CMB/ADHOC-BMS, dated 13.11.2019). Infrastructural support from the Department of Biosciences and Bioengineering at IIT Guwahati is acknowledged. SG was a student of the M.Tech program supported by the Department of Biotechnology, Govt. of India, which also provided her fellowship.
Author information
Authors and Affiliations
Contributions
UP and AML designed the experiments. UP and SG performed the experiments. UP and AML analysed the results. UP, SG, and AML wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research involving human and animal rights
The work involves cell line models. No human participants and animals are involved in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pal, U., Ghosh, S. & Limaye, A.M. DNA methylation in the upstream CpG island of the GPER locus and its relationship with GPER expression in colon cancer cell lines. Mol Biol Rep 47, 7547–7555 (2020). https://doi.org/10.1007/s11033-020-05817-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05817-5