Abstract
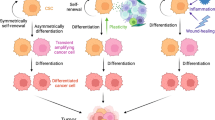
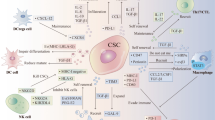
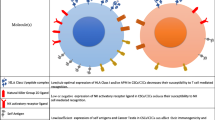
The multipotent, self renewing “cancer stem cells” (CSCs), a small population within tumor microenvironment facilitates transformed cells to grow and propagate within the body. The CSCs are discovered as resistant to the chemotherapeutic drug with distinct immunological characteristics. In recent years, immunologically targeting CSCs have emerged as an integral part of effective and successful cancer therapy. CSCs notably exhibit dysregulation in conventional sub-cellular sphingolipid metabolism. Recently, ceramide decaying enzymes have been shown to activate alternative ceramide signaling pathways leading to reduction in efficacy of the chemotherapeutic drugs. Therefore, a control over ceramide mediated modulations of CSCs offers an attractive dimension of effective cancer treatment strategy in future. In this review, we focused on the recent findings on broad spectrum of ceramide mediated signaling in CSCs within the tumor niche and their role in potential cancer immunotherapy.



Similar content being viewed by others
References
Kusoglu A, Biray Avci C (2019) Cancer stem cells: a brief review of the current status. Gene 681:80–85. https://doi.org/10.1016/j.gene.2018.09.052
Eun K, Ham SW, Kim H (2017) Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep 50(3):117–125. https://doi.org/10.5483/bmbrep.2017.50.3.222
Chang JC (2016) Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore) 95(1 Suppl 1):S20–25. https://doi.org/10.1097/MD.0000000000004766
Carnero A, Lleonart M (2016) The hypoxic microenvironment: a determinant of cancer stem cell evolution. BioEssays 38(Suppl 1):S65–74. https://doi.org/10.1002/bies.201670911
You L, Guo X, Huang Y (2018) Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med J 59(1):35–42. https://doi.org/10.3349/ymj.2018.59.1.35
Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, Montone K, Butzow R, Coukos G, Zhang L (2010) Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE 5(4):e10277. https://doi.org/10.1371/journal.pone.0010277
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. https://doi.org/10.1073/pnas.0530291100
Yu Z, Pestell TG, Lisanti MP, Pestell RG (2012) Cancer stem cells. Int J Biochem Cell Biol 44(12):2144–2151. https://doi.org/10.1016/j.biocel.2012.08.022
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648. https://doi.org/10.1038/367645a0
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63(18):5821–5828
Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, Zhao F, Jiang C, Hu W, Hu K, Gu N (2007) Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol 4(6):467–472
Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J (2011) Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol 64(11):937–946. https://doi.org/10.1136/jcp.2011.090456
Barkeer S, Chugh S, Batra SK, Ponnusamy MP (2018) Glycosylation of cancer stem cells: function in stemness, tumorigenesis, and metastasis. Neoplasia 20(8):813–825. https://doi.org/10.1016/j.neo.2018.06.001
Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, Consoli DP, Cabot MC (2008) A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J 22(7):2541–2551. https://doi.org/10.1096/fj.07-092981
Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS (2015) Concise review: targeting cancer stem cells using immunologic approaches. Stem Cells 33(7):2085–2092. https://doi.org/10.1002/stem.2039
Song M, Zang W, Zhang B, Cao J, Yang G (2012) GCS overexpression is associated with multidrug resistance of human HCT-8 colon cancer cells. J Exp Clin Cancer Res 31:23. https://doi.org/10.1186/1756-9966-31-23
Mobarak E, Haversen L, Manna M, Rutberg M, Levin M, Perkins R, Rog T, Vattulainen I, Boren J (2018) Glucosylceramide modifies the LPS-induced inflammatory response in macrophages and the orientation of the LPS/TLR4 complex in silico. Sci Rep 8(1):13600. https://doi.org/10.1038/s41598-018-31926-0
Oskouian B, Saba JD (2010) Cancer treatment strategies targeting sphingolipid metabolism. Adv Exp Med Biol 688:185–205. https://doi.org/10.1007/978-1-4419-6741-1_13
Ghosh S, Jawed JJ, Halder K, Banerjee S, Chowdhury BP, Saha A, Juin SK, Majumdar SB, Bose A, Baral R, Majumdar S (2018) TNFalpha mediated ceramide generation triggers cisplatin induced apoptosis in B16F10 melanoma in a PKCdelta independent manner. Oncotarget 9(102):37627–37646. https://doi.org/10.18632/oncotarget.26478
Ghosh S, Juin SK, Nandi P, Majumdar SB, Bose A, Baral R, Sil PC, Majumdar S (2020) PKCzeta mediated anti-proliferative effect of C2 ceramide on neutralization of the tumor microenvironment and melanoma regression. Cancer Immunol Immun 69(4):611–627. https://doi.org/10.1007/s00262-020-02492-0
Codd AS, Kanaseki T, Torigo T, Tabi Z (2018) Cancer stem cells as targets for immunotherapy. Immunology 153(3):304–314. https://doi.org/10.1111/imm.12866
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H (2011) Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA 108(30):12425–12430. https://doi.org/10.1073/pnas.1106645108
Codony-Servat J, Rosell R (2015) Cancer stem cells and immunoresistance: clinical implications and solutions. Transl Lung Cancer Res 4(6):689–703. https://doi.org/10.3978/j.issn.2218-6751.2015.12.11
Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J (2016) Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165(1):45–60. https://doi.org/10.1016/j.cell.2016.02.025
Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM, Galli R, Parmiani G, Maccalli C (2010) Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 16(3):800–813. https://doi.org/10.1158/1078-0432.CCR-09-2730
Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH (2010) Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res 70(2):697–708. https://doi.org/10.1158/0008-5472.CAN-09-1592
Guo M, Luo B, Pan M, Li M, Zhao F, Dou J (2020) MUC1 plays an essential role in tumor immunity of colorectal cancer stem cell vaccine. Int Immunopharmacol 85:106631. https://doi.org/10.1016/j.intimp.2020.106631
Alhabbab RY (2020) Targeting cancer stem cells by genetically engineered chimeric antigen receptor T cells. Front Genet 11:312. https://doi.org/10.3389/fgene.2020.00312
Khosravi N, Mokhtarzadeh A, Baghbanzadeh A, Hajiasgharzadeh K, Shahgoli VK, Hemmat N, Safarzadeh E, Baradaran B (2020) Immune checkpoints in tumor microenvironment and their relevance to the development of cancer stem cells. Life Sci. https://doi.org/10.1016/j.lfs.2020.118005
Valent P, Bauer K, Sadovnik I, Smiljkovic D, Ivanov D, Herrmann H, Filik Y, Eisenwort G, Sperr WR, Rabitsch W (2020) Cell-based and antibody-mediated immunotherapies directed against leukemic stem cells in acute myeloid leukemia: Perspectives and open issues. Stem Cells Transl Med. https://doi.org/10.1002/sctm.20-0147
Coderch L, Lopez O, de la Maza A, Parra JL (2003) Ceramides and skin function. Am J Clin Dermatol 4(2):107–129. https://doi.org/10.2165/00128071-200304020-00004
Henry B, Moller C, Dimanche-Boitrel MT, Gulbins E, Becker KA (2013) Targeting the ceramide system in cancer. Cancer Lett 332(2):286–294. https://doi.org/10.1016/j.canlet.2011.07.010
Dey R, Majumder N, Bhattacharjee S, Majumdar SB, Banerjee R, Ganguly S, Das P, Majumdar S (2007) Leishmania donovani-induced ceramide as the key mediator of Akt dephosphorylation in murine macrophages: role of protein kinase czeta and phosphatase. Infect Immun 75(5):2136–2142. https://doi.org/10.1128/IAI.01589-06
Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13(1):51–65. https://doi.org/10.1038/nrc3398
Pritzl CJ, Seo YJ, Xia C, Vijayan M, Stokes ZD, Hahm B (2015) A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J Immunol 194(9):4339–4349. https://doi.org/10.4049/jimmunol.1402672
Guenther GG, Edinger AL (2009) A new take on ceramide: starving cells by cutting off the nutrient supply. Cell Cycle 8(8):1122–1126. https://doi.org/10.4161/cc.8.8.8161
Raguz S, Yague E (2008) Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer 99(3):387–391. https://doi.org/10.1038/sj.bjc.6604510
Ahn EH, Yang H, Hsieh CY, Sun W, Chang CC, Schroeder JJ (2019) Evaluation of chemotherapeutic and cancer-protective properties of sphingosine and C2-ceramide in a human breast stem cell derived carcinogenesis model. Int J Oncol 54(2):655–664. https://doi.org/10.3892/ijo.2018.4641
Zhang Y, Wang H, Chen T, Wang H, Liang X, Zhang Y, Duan J, Qian S, Qiao K, Zhang L, Liu Y, Wang J (2020) C24-ceramide drives gallbladder cancer progression through directly targeting PIP4K2C to facilitate mTOR Signaling Activation. Hepatology. https://doi.org/10.1002/hep.31304
Ogretmen B (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18(1):33–50. https://doi.org/10.1038/nrc.2017.96
Woodcock J (2006) Sphingosine and ceramide signalling in apoptosis. IUBMB Life 58(8):462–466. https://doi.org/10.1080/15216540600871118
Bieberich E (2008) Ceramide signaling in cancer and stem cells. Future Lipidol 3(3):273–300. https://doi.org/10.2217/17460875.3.3.273
Korbelik M, Banath J, Sun J, Canals D, Hannun YA, Separovic D (2014) Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: cell surface exposure. Int Immunopharmacol 20(2):359–365. https://doi.org/10.1016/j.intimp.2014.03.016
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22(1):50–60. https://doi.org/10.1016/j.tcb.2011.09.003
Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, Alvarez SE (2016) Sphingosine-1 Phosphate: a new modulator of immune plasticity in the tumor microenvironment. Front Oncol 6:218. https://doi.org/10.3389/fonc.2016.00218
von Wenckstern H, Zimmermann K, Kleuser B (2006) The role of the lysophospholipid sphingosine 1-phosphate in immune cell biology. Arch Immunol Ther Exp (Warsz) 54(4):239–251. https://doi.org/10.1007/s00005-006-0028-9
Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, Brune B (2006) Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 108(5):1635–1642. https://doi.org/10.1182/blood-2006-04-014852
Weigert A, Tzieply N, von Knethen A, Johann AM, Schmidt H, Geisslinger G, Brune B (2007) Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell 18(10):3810–3819. https://doi.org/10.1091/mbc.e06-12-1096
Weis N, Weigert A, von Knethen A, Brune B (2009) Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 20(5):1280–1288. https://doi.org/10.1091/mbc.E08-10-1005
Lagadari M, Lehmann K, Ziemer M, Truta-Feles K, Berod L, Idzko M, Barz D, Kamradt T, Maghazachi AA, Norgauer J (2009) Sphingosine-1-phosphate inhibits the cytotoxic activity of NK cells via Gs protein-mediated signalling. Int J Oncol 34(1):287–294
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427(6972):355–360. https://doi.org/10.1038/nature02284
Riboni L, Abdel Hadi L, Navone SE, Guarnaccia L, Campanella R, Marfia G (2020) Sphingosine-1-phosphate in the tumor microenvironment: a signaling hub regulating cancer hallmarks. Cells. https://doi.org/10.3390/cells9020337
Bai A, Kokkotou E, Zheng Y, Robson SC (2015) Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis 6:e1828. https://doi.org/10.1038/cddis.2015.178
Herz J, Pardo J, Kashkar H, Schramm M, Kuzmenkina E, Bos E, Wiegmann K, Wallich R, Peters PJ, Herzig S, Schmelzer E, Kronke M, Simon MM, Utermohlen O (2009) Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat Immunol 10(7):761–768. https://doi.org/10.1038/ni.1757
Schneider-Schaulies J, Beyersdorf N (2018) CD4+ Foxp3+ regulatory T cell-mediated immunomodulation by anti-depressants inhibiting acid sphingomyelinase. Biol Chem 399(10):1175–1182. https://doi.org/10.1515/hsz-2018-0159
Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, Robson SC (2014) CD39 and CD161 modulate Th17 responses in crohn's disease. J Immunol 193(7):3366–3377. https://doi.org/10.4049/jimmunol.1400346
Sofi MH, Heinrichs J, Dany M, Nguyen H, Dai M, Bastian D, Schutt S, Wu Y, Daenthanasanmak A, Gencer S, Zivkovic A, Szulc Z, Stark H, Liu C, Chang YJ, Ogretmen B, Yu XZ (2017) Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. https://doi.org/10.1172/jci.insight.91701
Liu F, Li X, Lu C, Bai A, Bielawski J, Bielawska A, Marshall B, Schoenlein PV, Lebedyeva IO, Liu K (2016) Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget 7(51):83907–83925. https://doi.org/10.18632/oncotarget.13438
Yin J, Miyazaki K, Shaner RL, Merrill AH Jr, Kannagi R (2010) Altered sphingolipid metabolism induced by tumor hypoxia - new vistas in glycolipid tumor markers. FEBS Lett 584(9):1872–1878. https://doi.org/10.1016/j.febslet.2009.11.019
Liu YY, Hill RA, Li YT (2013) Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance. Adv Cancer Res 117:59–89. https://doi.org/10.1016/B978-0-12-394274-6.00003-0
Chaturvedi P, Gilkes DM, Takano N, Semenza GL (2014) Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci USA 111(20):E2120–2129. https://doi.org/10.1073/pnas.1406655111
Kang MS, Ahn KH, Kim SK, Jeon HJ, Ji JE, Choi JM, Jung KM, Jung SY, Kim DK (2010) Hypoxia-induced neuronal apoptosis is mediated by de novo synthesis of ceramide through activation of serine palmitoyltransferase. Cell Signal 22(4):610–618. https://doi.org/10.1016/j.cellsig.2009.11.015
Hosain SB, Khiste SK, Uddin MB, Vorubindi V, Ingram C, Zhang S, Hill RA, Gu X, Liu YY (2016) Inhibition of glucosylceramide synthase eliminates the oncogenic function of p53 R273H mutant in the epithelial-mesenchymal transition and induced pluripotency of colon cancer cells. Oncotarget 7(37):60575–60592. https://doi.org/10.18632/oncotarget.11169
Ichikawa S, Hirabayashi Y (1998) Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol 8(5):198–202. https://doi.org/10.1016/s0962-8924(98)01249-5
Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD (2012) O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11(1):62–74. https://doi.org/10.1016/j.stem.2012.03.001
Liu Y, Xie KM, Yang GQ, Bai XM, Shi YP, Mu HJ, Qiao WZ, Zhang B, Xie P (2010) GCS induces multidrug resistance by regulating apoptosis-related genes in K562/AO2 cell line. Cancer Chemother Pharmacol 66(3):433–439. https://doi.org/10.1007/s00280-009-1177-4
Xie P, Shen YF, Shi YP, Ge SM, Gu ZH, Wang J, Mu HJ, Zhang B, Qiao WZ, Xie KM (2008) Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leuk Res 32(3):475–480. https://doi.org/10.1016/j.leukres.2007.07.006
Madigan JP, Robey RW, Poprawski JE, Huang H, Clarke CJ, Gottesman MM, Cabot MC, Rosenberg DW (2020) A role for ceramide glycosylation in resistance to oxaliplatin in colorectal cancer. Exp Cell Res 388(2):111860. https://doi.org/10.1016/j.yexcr.2020.111860
Salustiano EJ, da Costa KM, Freire-de-Lima L, Mendonca-Previato L, Previato JO (2020) Inhibition of glycosphingolipid biosynthesis reverts multidrug resistance by differentially modulating ABC transporters in chronic myeloid leukemias. J Biol Chem 295(19):6457–6471. https://doi.org/10.1074/jbc.RA120.013090
Zhang X, Li J, Qiu Z, Gao P, Wu X, Zhou G (2009) Co-suppression of MDR1 (multidrug resistance 1) and GCS (glucosylceramide synthase) restores sensitivity to multidrug resistance breast cancer cells by RNA interference (RNAi). Cancer Biol Ther 8(12):1117–1121. https://doi.org/10.4161/cbt.8.12.8374
Patwardhan GA, Zhang QJ, Yin D, Gupta V, Bao J, Senkal CE, Ogretmen B, Cabot MC, Shah GV, Sylvester PW, Jazwinski SM, Liu YY (2009) A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PLoS ONE 4(9):e6938. https://doi.org/10.1371/journal.pone.0006938
Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, Mehendale H, Cabot MC, Li YT, Jazwinski SM (2010) Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer 9:145. https://doi.org/10.1186/1476-4598-9-145
Escoll M, Gargini R, Cuadrado A, Anton IM, Wandosell F (2017) Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene 36(25):3515–3527. https://doi.org/10.1038/onc.2016.518
Liu YY, Patwardhan GA, Bhinge K, Gupta V, Gu X, Jazwinski SM (2011) Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Res 71(6):2276–2285. https://doi.org/10.1158/0008-5472.CAN-10-3107
Zhang X, Wu X, Su P, Gao Y, Meng B, Sun Y, Li L, Zhou Z, Zhou G (2012) Doxorubicin influences the expression of glucosylceramide synthase in invasive ductal breast cancer. PLoS ONE 7(11):e48492. https://doi.org/10.1371/journal.pone.0048492
Uchida Y, Itoh M, Taguchi Y, Yamaoka S, Umehara H, Ichikawa S, Hirabayashi Y, Holleran WM, Okazaki T (2004) Ceramide reduction and transcriptional up-regulation of glucosylceramide synthase through doxorubicin-activated Sp1 in drug-resistant HL-60/ADR cells. Cancer Res 64(17):6271–6279. https://doi.org/10.1158/0008-5472.CAN-03-1476
Gupta V, Bhinge KN, Hosain SB, Xiong K, Gu X, Shi R, Ho MY, Khoo KH, Li SC, Li YT, Ambudkar SV, Jazwinski SM, Liu YY (2012) Ceramide glycosylation by glucosylceramide synthase selectively maintains the properties of breast cancer stem cells. J Biol Chem 287(44):37195–37205. https://doi.org/10.1074/jbc.M112.396390
Doan NB, Alhajala H, Al-Gizawiy MM, Mueller WM, Rand SD, Connelly JM, Cochran EJ, Chitambar CR, Clark P, Kuo J, Schmainda KM, Mirza SP (2017) Acid ceramidase and its inhibitors: a de novo drug target and a new class of drugs for killing glioblastoma cancer stem cells with high efficiency. Oncotarget 8(68):112662–112674. https://doi.org/10.18632/oncotarget.22637
White-Gilbertson S, Lu P, Norris JS, Voelkel-Johnson C (2019) Genetic and pharmacological inhibition of acid ceramidase prevents asymmetric cell division by neosis. J Lipid Res 60(7):1225–1235. https://doi.org/10.1194/jlr.M092247
Lai M, Realini N, La Ferla M, Passalacqua I, Matteoli G, Ganesan A, Pistello M, Mazzanti CM, Piomelli D (2017) Complete acid ceramidase ablation prevents cancer-initiating cell formation in melanoma cells. Sci Rep 7(1):7411. https://doi.org/10.1038/s41598-017-07606-w
Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10(7):489–503. https://doi.org/10.1038/nrc2875
Hirata N, Yamada S, Shoda T, Kurihara M, Sekino Y, Kanda Y (2014) Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent notch activation. Nat Commun 5:4806. https://doi.org/10.1038/ncomms5806
Sordillo LA, Sordillo PP, Helson L (2016) Sphingosine kinase inhibitors as maintenance therapy of glioblastoma after ceramide-induced response. Anticancer Res 36(5):2085–2095
Ng ML, Yarla NS, Menschikowski M, Sukocheva OA (2018) Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J Stem Cells 10(9):119–133. https://doi.org/10.4252/wjsc.v10.i9.119
Wang YC, Tsai CF, Chuang HL, Chang YC, Chen HS, Lee JN, Tsai EM (2016) Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget 7(20):29563–29576. https://doi.org/10.18632/oncotarget.9007
Gomez-Munoz A (2018) The role of ceramide 1-phosphate in tumor cell survival and dissemination. Adv Cancer Res 140:217–234. https://doi.org/10.1016/bs.acr.2018.04.012
Kuc N, Doermann A, Shirey C, Lee DD, Lowe CW, Awasthi N, Schwarz RE, Stahelin RV, Schwarz MA (2018) Pancreatic ductal adenocarcinoma cell secreted extracellular vesicles containing ceramide-1-phosphate promote pancreatic cancer stem cell motility. Biochem Pharmacol 156:458–466. https://doi.org/10.1016/j.bcp.2018.09.017
Zhong L, Kong JN, Dinkins MB, Leanhart S, Zhu Z, Spassieva SD, Qin H, Lin HP, Elsherbini A, Wang R, Jiang X, Nikolova-Karakashian M, Wang G, Bieberich E (2018) Increased liver tumor formation in neutral sphingomyelinase-2-deficient mice. J Lipid Res 59(5):795–804. https://doi.org/10.1194/jlr.M080879
Yeh SC, Wang PY, Lou YW, Khoo KH, Hsiao M, Hsu TL, Wong CH (2016) Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc Natl Acad Sci USA 113(20):5592–5597. https://doi.org/10.1073/pnas.1604721113
Saygin C, Wiechert A, Rao VS, Alluri R, Connor E, Thiagarajan PS, Hale JS, Li Y, Chumakova A, Jarrar A, Parker Y, Lindner DJ, Nagaraj AB, Kim JJ, DiFeo A, Abdul-Karim FW, Michener C, Rose PG, DeBernardo R, Mahdi H, McCrae KR, Lin F, Lathia JD, Reizes O (2017) CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J Exp Med 214(9):2715–2732. https://doi.org/10.1084/jem.20170438
Xu JX, Morii E, Liu Y, Nakamichi N, Ikeda J, Kimura H, Aozasa K (2007) High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: high expression of CD55 as a novel character for side-population. Exp Cell Res 313(9):1877–1885. https://doi.org/10.1016/j.yexcr.2007.03.006
Cruz AF, Fonseca NA, Gonçalves N, Moura V, Simões S, Moreira JN (2019) Differential effect of liposomal C6-ceramide/doxorubicin targeted to nucleolin and conventional combinations against triple negative breast cancer stem cells. Cancer Res 79:3622
Su X, Song H, Niu F, Yang K, Kou G, Wang X, Chen H, Li W, Guo S, Li J, Li B, Feng SS, Jiang J, Yin C, Gao J (2015) Co-delivery of doxorubicin and PEGylated C16-ceramide by nanoliposomes for enhanced therapy against multidrug resistance. Nanomedicine (Lond) 10(13):2033–2050. https://doi.org/10.2217/nnm.15.50
Wang M, Xie F, Wen X, Chen H, Zhang H, Liu J, Zhang H, Zou H, Yu Y, Chen Y, Sun Z, Wang X, Zhang G, Yin C, Sun D, Gao J, Jiang B, Zhong Y, Lu Y (2017) Therapeutic PEG-ceramide nanomicelles synergize with salinomycin to target both liver cancer cells and cancer stem cells. Nanomedicine (Lond) 12(9):1025–1042. https://doi.org/10.2217/nnm-2016-0408
Banerjee S, Halder K, Ghosh S, Bose A, Majumdar S (2015) The combination of a novel immunomodulator with a regulatory T cell suppressing antibody (DTA-1) regress advanced stage B16F10 solid tumor by repolarizing tumor associated macrophages in situ. Oncoimmunology 4(3):e995559. https://doi.org/10.1080/2162402X.2014.995559
Acknowledgements
We are very much thankful to the director Bose institute, Kolkata for providing us the working environment and all the necessary facilities to support the research work.
Funding
The work was supported by the Council of Scientific and Industrial Research (CSIR), India (Grant No.: 09/015(0475)2015-EMR-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal rights
No human participants and/or animals are involved for research.
Informed consent
Informed consent obtained from all individual participants was included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, S., Juin, S.K. & Majumdar, S. Cancer stem cells and ceramide signaling: the cutting edges of immunotherapy. Mol Biol Rep 47, 8101–8111 (2020). https://doi.org/10.1007/s11033-020-05790-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05790-z