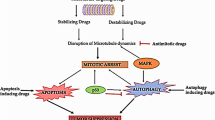
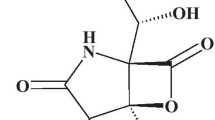
Abstract
Marine invertebrates are extremely diverse, largely productive, untapped oceanic resources with chemically unique bioactive lead compound contributing a wide range of screening for the discovery of anticancer compounds. The lead compounds have unfurled an extensive array of pharmacological properties owing to the presence of polyphenols, alkaloids, terpenoids and other secondary metabolites. The antioxidant, immunomodulatory and anti-tumor activities exhibited, are possibly regulated by the apoptosis induction, scavenging of ROS and modulation of cellular signaling pathways to defy the cellular deafness during carcinogenesis. Despite the enriched bioactive compounds, the marine invertebrates are largely unexplored as identification, screening, pre-clinical and clinical assessment of lead compounds and their synthetic analogs remain a major task to be solved. In the current review, we focus on the principle strategy and underlying mechanisms deployed by the bioactive anticancer compounds derived from marine invertebrates to combat cancer with special insight into the cell death mechanism.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Bhutia SK, Panda PK, Sinha N, Praharaj PP, Bhol CS, Panigrahi DP, Mahapatra KK, Saha S, Patra S, Mishra SR, Behera BP, Patil S, Maiti TK (2019) Plant lectins in cancer therapeutics: targeting apoptosis and autophagy-dependent cell death. Pharmacol Res 144:8–18. https://doi.org/10.1016/j.phrs.2019.04.001
Patra S, Panda PK, Naik PP, Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Padhi P, Jena M, Patil S, Patra SK, Bhutia SK (2020) Terminalia bellirica extract induces anticancer activity through modulation of apoptosis and autophagy in oral squamous cell carcinoma. Food Chem Toxicol 136:111073. https://doi.org/10.1016/j.fct.2019.111073
Patra S, Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Mishra SR, Behera BP, Jena M, Bhutia SK (2019) Dysregulation of histone deacetylases in carcinogenesis and tumor progression: a possible link to apoptosis and autophagy. 76(17):3263–3282. https://doi.org/10.1007/s00018-019-03098-1
Patra S, Mishra SR, Behera BP, Mahapatra KK, Panigrahi DP, Bhol CS, Praharaj PP, Sethi G, Patra SK, Bhutia SK (2020) Autophagy-modulating phytochemicals in cancer therapeutics: current evidences and future perspectives. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.05.008
Gomes NG, Dasari R, Chandra S, Kiss R, Kornienko A (2016) Marine invertebrate metabolites with anticancer activities: solutions to the “Supply Problem.” Mar Drugs. https://doi.org/10.3390/md14050098
Ruiz-Torres V, Encinar JA, Herranz-Lopez M, Perez-Sanchez A, Galiano V, Barrajon-Catalan E, Micol V (2017) An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small-molecule cancer drugs. Molecules. https://doi.org/10.3390/molecules22071037
Hu Y, Chen J, Hu G, Yu J, Zhu X, Lin Y, Chen S, Yuan J (2015) Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar Drugs 13(1):202–221. https://doi.org/10.3390/md13010202
Gogineni V, Hamann MT (2018) Marine natural product peptides with therapeutic potential: chemistry, biosynthesis, and pharmacology. Biochim Biophys Acta 1862(1):81–196. https://doi.org/10.1016/j.bbagen.2017.08.014
Agarwal V, Blanton JM, Podell S, Taton A, Schorn MA, Busch J, Lin Z, Schmidt EW, Jensen PR, Paul VJ, Biggs JS, Golden JW, Allen EE, Moore BS (2017) Metagenomic discovery of polybrominated diphenyl ether biosynthesis by marine sponges. Nat Chem Biol 13(5):537–543. https://doi.org/10.1038/nchembio.2330
Calcabrini C, Catanzaro E, Bishayee A, Turrini E (2017) Marine sponge natural products with anticancer potential: an updated review. Mar Drugs 15(10):310. https://doi.org/10.3390/md15100310
Song X, Xiong Y, Qi X, Tang W, Dai J, Gu Q, Li J (2018) Molecular targets of active anticancer compounds derived from marine sources. Mar Drugs. https://doi.org/10.3390/md16050175
Liao CH, Lai IC, Kuo HC, Chuang SE, Lee HL, Whang-Peng J, Yao CJ, Lai GM (2019) Epigenetic modification and differentiation induction of malignant glioma cells by Oligo-Fucoidan. Mar Drugs. https://doi.org/10.3390/md17090525
Van Soest RW, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ, Santodomingo N, Vanhoorne B, Kelly M, Hooper JN (2012) Global diversity of sponges (Porifera). PLoS ONE 7(4):e35105. https://doi.org/10.1371/journal.pone.0035105
Dembitsky VM (2002) Bromo- and iodo-containing alkaloids from marine microorganisms and sponges. Bioorg Khim 28(3):196–208
Nakazawa H, Kitano K, Cioca D, Ishikawa M, Ueno M, Ishida F, Kiyosawa K (2000) Induction of polyploidization by jaspamide in HL-60 cells. Acta Haematol 104(2–3):65–71. https://doi.org/10.1159/000039754
Suarez-Jimenez GM, Burgos-Hernandez A, Ezquerra-Brauer JM (2012) Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Mar Drugs 10(5):963–986. https://doi.org/10.3390/md10050963
Wang L, Dong C, Li X, Han W, Su X (2017) Anticancer potential of bioactive peptides from animal sources (Review). Oncol Rep 38(2):637–651. https://doi.org/10.3892/or.2017.5778
Odaka C, Sanders ML, Crews P (2000) Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin Diagn Lab Immunol 7(6):947–952. https://doi.org/10.1128/cdli.7.6.947-952.2000
Plaza A, Bifulco G, Keffer JL, Lloyd JR, Baker HL, Bewley CA (2009) Celebesides A-C and theopapuamides B-D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J Org Chem 74(2):504–512. https://doi.org/10.1021/jo802232u
Plaza A, Gustchina E, Baker HL, Kelly M, Bewley CA (2007) Mirabamides A-D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J Nat Prod 70(11):1753–1760. https://doi.org/10.1021/np070306k
Hamada Y, Shioiri T (2005) Recent progress of the synthetic studies of biologically active marine cyclic peptides and depsipeptides. Chem Rev 105(12):4441–4482. https://doi.org/10.1021/cr0406312
Freitas VM, Rangel M, Bisson LF, Jaeger RG, Machado-Santelli GM (2008) The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J Cell Physiol 216(3):583–594. https://doi.org/10.1002/jcp.21432
Zampella A, Sepe V, Luciano P, Bellotta F, Monti MC, D’Auria MV, Jepsen T, Petek S, Adeline MT, Laprevote O, Aubertin AM, Debitus C, Poupat C, Ahond A (2008) Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J Org Chem 73(14):5319–5327. https://doi.org/10.1021/jo800583b
Kang HK, Choi MC, Seo CH, Park Y (2018) Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int J Mol Sci. https://doi.org/10.3390/ijms19030919
Hamada T, Matsunaga S, Yano G, Fusetani N (2005) Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc 127(1):110–118. https://doi.org/10.1021/ja045749e
Giordano D, Costantini M, Coppola D, Lauritano C, Nunez Pons L, Ruocco N, di Prisco G, Ianora A, Verde C (2018) Biotechnological applications of bioactive peptides from marine sources. Adv Microb Physiol 73:171–220. https://doi.org/10.1016/bs.ampbs.2018.05.002
Fang WY, Dahiya R, Qin HL, Mourya R, Maharaj S (2016) Natural Proline-Rich Cyclopolypeptides from Marine Organisms: Chemistry. Mar Drugs, Synthetic Methodologies and Biological Status. https://doi.org/10.3390/md14110194
Coleman RA, Pugh BF (1995) Evidence for functional binding and stable sliding of the TATA binding protein on nonspecific DNA. J Biol Chem 270(23):13850–13859. https://doi.org/10.1074/jbc.270.23.13850
Kuznetsov G, TenDyke K, Towle MJ, Cheng H, Liu J, Marsh JP, Schiller SE, Spyvee MR, Yang H, Seletsky BM, Shaffer CJ, Marceau V, Yao Y, Suh EM, Campagna S, Fang FG, Kowalczyk JJ, Littlefield BA (2009) Tubulin-based antimitotic mechanism of E7974, a novel analogue of the marine sponge natural product hemiasterlin. Mol Cancer Ther 8(10):2852–2860. https://doi.org/10.1158/1535-7163.mct-09-0301
Williams DE, Austin P, Diaz-Marrero AR, Soest RV, Matainaho T, Roskelley CD, Roberge M, Andersen RJ (2005) Neopetrosiamides, peptides from the marine sponge Neopetrosia sp. that inhibit amoeboid invasion by human tumor cells. Org Lett 7(19):4173–4176. https://doi.org/10.1021/ol051524c
Nakao Y, Kawatsu S, Okamoto C, Okamoto M, Matsumoto Y, Matsunaga S, van Soest RW, Fusetani N (2008) Ciliatamides A-C, bioactive lipopeptides from the deep-sea sponge Aaptos ciliata. J Nat Prod 71(3):469–472. https://doi.org/10.1021/np8000317
Nakao Y, Yoshida S, Matsunaga S, Shindoh N, Terada Y, Nagai K, Yamashita JK, Ganesan A, van Soest RW, Fusetani N (2006) Azumamides A-E: histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew Chem 45(45):7553–7557. https://doi.org/10.1002/anie.200602047
Li WL, Yi YH, Wu HM, Xu QZ, Tang HF, Zhou DZ, Lin HW, Wang ZH (2003) Isolation and structure of the cytotoxic cycloheptapeptide phakellistatin 13. Journal of natural products 66(1):146–148. https://doi.org/10.1021/np020223y
Meli A, Tedesco C, Della Sala G, Schettini R, Albericio F, De Riccardis F, Izzo I (2017) Phakellistatins: an underwater unsolved puzzle. Mar Drugs. https://doi.org/10.3390/md15030078
Pettit GR, Tan R (2005) Isolation and structure of phakellistatin 14 from the Western Pacific marine sponge Phakellia sp. J Nat Prod 68(1):60–63. https://doi.org/10.1021/np040092w
Williams DE, Yu K, Behrisch HW, Van Soest R, Andersen RJ (2009) Rolloamides A and B, cytotoxic cyclic heptapeptides isolated from the Caribbean marine sponge Eurypon laughlini. J Nat Prod 72(7):1253–1257. https://doi.org/10.1021/np900121m
Arai M, Yamano Y, Fujita M, Setiawan A, Kobayashi M (2012) Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorg Med Chem Lett 22(4):1818–1821. https://doi.org/10.1016/j.bmcl.2011.10.023
Woo JK, Jeon JE, Kim CK, Sim CJ, Oh DC, Oh KB, Shin J (2013) Gombamide A, a cyclic thiopeptide from the sponge Clathria gombawuiensis. J Nat Prod 76(7):1380–1383. https://doi.org/10.1021/np4003367
Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH (2005) Marine natural products as anticancer drugs. Mol Cancer Ther 4(2):333–342
Lee YJ, Yoo SJ, Kang JS, Yun J, Shin HJ, Lee JS, Lee HS (2013) Cytotoxic petrosiacetylenes from the marine sponge Petrosia sp. Lipids 48(1):87–91. https://doi.org/10.1007/s11745-012-3727-5
Tsukamoto S, Yamanokuchi R, Yoshitomi M, Sato K, Ikeda T, Rotinsulu H, Mangindaan RE, de Voogd NJ, van Soest RW, Yokosawa H (2010) Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorg Med Chem Lett 20(11):3341–3343. https://doi.org/10.1016/j.bmcl.2010.04.029
Li F, Peifer C, Janussen D, Tasdemir D (2019) New discorhabdin alkaloids from the Antarctic deep-sea sponge Latrunculia biformis. Mar Drugs. https://doi.org/10.3390/md17080439
Hu JF, Fan H, Xiong J, Wu SB (2011) Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem Rev 111(9):5465–5491. https://doi.org/10.1021/cr100435g
Broker LE, Huisman C, Ferreira CG, Rodriguez JA, Kruyt FA, Giaccone G (2002) Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone B in non-small cell lung cancer cells. Cancer Res 62(14):4081–4088
Martello LA, McDaid HM, Regl DL, Yang CP, Meng D, Pettus TR, Kaufman MD, Arimoto H, Danishefsky SJ, Smith AB 3rd, Horwitz SB (2000) Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin Cancer Res 6(5):1978–1987
Xu Q, Huang KC, Tendyke K, Marsh J, Liu J, Qiu D, Littlefield BA, Nomoto K, Atasoylu O, Risatti CA, Sperry JB, Smith AB 3rd (2011) In vitro and in vivo anticancer activity of (+)-spongistatin 1. Anticancer Res 31(9):2773–2779
Schyschka L, Rudy A, Jeremias I, Barth N, Pettit GR, Vollmar AM (2008) Spongistatin 1: a new chemosensitizing marine compound that degrades XIAP. Leukemia 22(9):1737–1745. https://doi.org/10.1038/leu.2008.146
Schneiders UM, Schyschka L, Rudy A, Vollmar AM (2009) BH3-only proteins Mcl-1 and Bim as well as endonuclease G are targeted in spongistatin 1-induced apoptosis in breast cancer cells. Mol Cancer Ther 8(10):2914–2925. https://doi.org/10.1158/1535-7163.mct-08-1179
Rothmeier AS, Ischenko I, Joore J, Garczarczyk D, Furst R, Bruns CJ, Vollmar AM, Zahler S (2009) Investigation of the marine compound spongistatin 1 links the inhibition of PKCalpha translocation to nonmitotic effects of tubulin antagonism in angiogenesis. FASEB J 23(4):1127–1137. https://doi.org/10.1096/fj.08-117127
Kawano S, Ito K, Yahata K (2019) A landmark in drug discovery based on complex natural product synthesis. Sci Rep 9(1):8656. https://doi.org/10.1038/s41598-019-45001-9
Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA (2004) Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res 64(16):5760–5766. https://doi.org/10.1158/0008-5472.can-04-1169
Mariottini GL, Pane L (2013) Cytotoxic and cytolytic cnidarian venoms. A review on health implications and possible therapeutic applications. Toxins 6(1):108–151. https://doi.org/10.3390/toxins6010108
Turk T, Kem WR (2009) The phylum Cnidaria and investigations of its toxins and venoms until 1990. Toxicon 54(8):1031–1037. https://doi.org/10.1016/j.toxicon.2009.06.031
Rocha J, Peixe L, Gomes NC, Calado R (2011) Cnidarians as a source of new marine bioactive compounds—an overview of the last decade and future steps for bioprospecting. Mar Drugs 9(10):1860–1886. https://doi.org/10.3390/md9101860
Su JH, Ahmed AF, Sung PJ, Chao CH, Kuo YH, Sheu JH (2006) Manaarenolides A-I, diterpenoids from the soft coral Sinularia manaarensis. J Nat Prod 69(8):1134–1139. https://doi.org/10.1021/np050483q
Li G, Zhang Y, Deng Z, van Ofwegen L, Proksch P, Lin W (2005) Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J Nat Prod 68(5):649–652. https://doi.org/10.1021/np040197z
Chen BW, Chao CH, Su JH, Tsai CW, Wang WH, Wen ZH, Huang CY, Sung PJ, Wu YC, Sheu JH (2011) Klysimplexins I-T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org Biomol Chem 9(3):834–844. https://doi.org/10.1039/c0ob00351d
Chen BW, Chao CH, Su JH, Wen ZH, Sung PJ, Sheu JH (2010) Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org Biomol Chem 8(10):2363–2366. https://doi.org/10.1039/b926353e
El-Gamal AA, Chiang CY, Huang SH, Wang SK, Duh CY (2005) Xenia diterpenoids from the formosan soft coral Xenia blumi. J Nat Prod 68(9):1336–1340. https://doi.org/10.1021/np058047r
Duh CY, El-Gamal AA, Chu CJ, Wang SK, Dai CF (2002) New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea. J Nat Prod 65(11):1535–1539. https://doi.org/10.1021/np0201873
Su CC, Wong BS, Chin C, Wu YJ, Su JH (2013) Oxygenated cembranoids from the soft coral Sinularia flexibilis. Int J Mol Sci 14(2):4317–4325. https://doi.org/10.3390/ijms14024317
Marrero J, Rodriguez AD, Baran P, Raptis RG, Sanchez JA, Ortega-Barria E, Capson TL (2004) Bielschowskysin, a gorgonian-derived biologically active diterpene with an unprecedented carbon skeleton. Org Lett 6(10):1661–1664. https://doi.org/10.1021/ol049495d
Rodriguez II, Rodriguez AD, Zhao H (2009) Aberrarone: a gorgonian-derived diterpene from Pseudopterogorgia elisabethae. J Org Chem 74(19):7581–7584. https://doi.org/10.1021/jo901578r
Kate AS, Pearson JK, Ramanathan B, Richard K, Kerr RG (2009) Isolation, biomimetic synthesis, and cytotoxic activity of bis(pseudopterane) amines. J Nat Prod 72(7):1331–1334. https://doi.org/10.1021/np8008144
Nam NH, Tung PT, Ngoc NT, Hanh TT, Thao NP, Thanh NV, Cuong NX, Thao do T, Huong TT, Thung do C, Kiem PV, Kim YH, Minh CV (2015) Cytotoxic biscembranoids from the soft coral Sarcophyton pauciplicatum. Chem Pharm Bull 63(8):636–640. https://doi.org/10.1248/cpb.c15-00273
Chai XY, Sun JF, Tang LY, Yang XW, Li YQ, Huang H, Zhou XF, Yang B, Liu Y (2010) A novel cyclopentene derivative and a polyhydroxylated steroid from a South China Sea gorgonian Menella sp. Chem Pharm Bull 58(10):1391–1394. https://doi.org/10.1248/cpb.58.1391
Ramos-Enriquez MA, Vargas-Romero K, Rarova L, Strnad M, Iglesias-Arteaga MA (2017) Synthesis and in vitro anticancer activity of 23(23’)E-benzylidenespirostanols derived from steroid sapogenins. Steroids 128:85–88. https://doi.org/10.1016/j.steroids.2017.08.017
Sheu JH, Hung KC, Wang GH, Duh CY (2000) New cytotoxic sesquiterpenes from the gorgonian Isis hippuris. J Nat Prod 63(12):1603–1607. https://doi.org/10.1021/np000271n
Chao CH, Huang LF, Wu SL, Su JH, Huang HC, Sheu JH (2005) Steroids from the gorgonian Isis hippuris. J Nat Prod 68(9):1366–1370. https://doi.org/10.1021/np050200u
Huang HC, Chao CH, Kuo YH, Sheu JH (2009) Crassocolides G-M, cembranoids from the Formosan soft coral Sarcophyton crassocaule. Chem Biodivers 6(8):1232–1242. https://doi.org/10.1002/cbdv.200800142
Lin WY, Chen BW, Huang CY, Wen ZH, Sung PJ, Su JH, Dai CF, Sheu JH (2014) Bioactive cembranoids, sarcocrassocolides P-R, from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar Drugs 12(2):840–850. https://doi.org/10.3390/md12020840
Grote D, Hanel F, Dahse HM, Seifert K (2008) Capnellenes from the soft coral Dendronephthya rubeola. Chem Biodivers 5(9):1683–1693. https://doi.org/10.1002/cbdv.200890157
Hermeking H (2003) The MYC oncogene as a cancer drug target. Curr Cancer Drug Targets 3(3):163–175
Watanabe K, Sekine M, Takahashi H, Iguchi K (2001) New halogenated marine prostanoids with cytotoxic activity from the Okinawan soft coral Clavularia viridis. J Nat Prod 64(11):1421–1425. https://doi.org/10.1021/np010244c
Shen YC, Cheng YB, Lin YC, Guh JH, Teng CM, Ko CL (2004) New prostanoids with cytotoxic activity from Taiwanese octocoral Clavularia viridis. J Nat Prod 67(4):542–546. https://doi.org/10.1021/np030435a
Cheng SY, Huang KJ, Wang SK, Wen ZH, Hsu CH, Dai CF, Duh CY (2009) New terpenoids from the soft corals Sinularia capillosa and Nephthea chabroli. Org Lett 11(21):4830–4833. https://doi.org/10.1021/ol901864d
Verbitski SM, Mullally JE, Fitzpatrick FA, Ireland CM (2004) Punaglandins, chlorinated prostaglandins, function as potent Michael receptors to inhibit ubiquitin isopeptidase activity. J Med Chem 47(8):2062–2070. https://doi.org/10.1021/jm030448l
Iwashima M, Nara K, Nakamichi Y, Iguchi K (2001) Three new chlorinated marine steroids, yonarasterols G, H and I, isolated from the okinawan soft coral, Clavularia viridis. Steroids 66(1):25–32. https://doi.org/10.1016/s0039-128x(00)00144-6
Ciavatta ML, Lefranc F, Carbone M, Mollo E, Gavagnin M, Betancourt T, Dasari R, Kornienko A, Kiss R (2017) Marine mollusk-derived agents with antiproliferative activity as promising anticancer agents to overcome chemotherapy resistance. Med Res Rev 37(4):702–801. https://doi.org/10.1002/med.21423
Maderna A, Leverett CA (2015) Recent advances in the development of new auristatins: structural modifications and application in antibody drug conjugates. Mol Pharm 12(6):1798–1812. https://doi.org/10.1021/mp500762u
Pettit RK, Pettit GR, Hazen KC (1998) Specific activities of dolastatin 10 and peptide derivatives against Cryptococcus neoformans. Antimicrob Agents Chemother 42(11):2961–2965. https://doi.org/10.1128/aac.42.11.2961
Bai R, Pettit GR, Hamel E (1990) Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol 39(12):1941–1949. https://doi.org/10.1016/0006-2952(90)90613-p
Thornburg CC, Thimmaiah M, Shaala LA, Hau AM, Malmo JM, Ishmael JE, Youssef DT, McPhail KL (2011) Cyclic depsipeptides, grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya cyanobacterium. J Nat Prod 74(8):1677–1685. https://doi.org/10.1021/np200270d
Pettit GR, Xu JP, Hogan F, Williams MD, Doubek DL, Schmidt JM, Cerny RL, Boyd MR (1997) Isolation and structure of the human cancer cell growth inhibitory cyclodepsipeptide dolastatin 16. J Nat Prod 60(8):752–754. https://doi.org/10.1021/np9700230
Bai R, Covell DG, Liu C, Ghosh AK, Hamel E (2002) (-)-Doliculide, a new macrocyclic depsipeptide enhancer of actin assembly. J Biol Chem 277(35):32165–32171. https://doi.org/10.1074/jbc.M205076200
Foerster F, Chen T, Altmann KH, Vollmar AM (2016) Actin-binding doliculide causes premature senescence in p53 wild type cells. Bioorg Med Chem 24(2):123–129. https://doi.org/10.1016/j.bmc.2015.11.042
Darro F, Decaestecker C, Gaussin JF, Mortier S, Van Ginckel R, Kiss R (2005) Are syngeneic mouse tumor models still valuable experimental models in the field of anti-cancer drug discovery? Int J Oncol 27(3):607–616
Sato S, Murata A, Orihara T, Shirakawa T, Suenaga K, Kigoshi H, Uesugi M (2011) Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem Biol 18(1):131–139. https://doi.org/10.1016/j.chembiol.2010.10.017
Gao J, Hamann MT (2011) Chemistry and biology of kahalalides. Chem Rev 111(5):3208–3235. https://doi.org/10.1021/cr100187n
Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A (2003) Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther 2(9):863–872
Braet F, Spector I, Shochet N, Crews P, Higa T, Menu E, de Zanger R, Wisse E (2002) The new anti-actin agent dihydrohalichondramide reveals fenestrae-forming centers in hepatic endothelial cells. BMC Cell Biol 3:7
Bae SY, Kim GD, Jeon JE, Shin J, Lee SK (2013) Anti-proliferative effect of (19Z)-halichondramide, a novel marine macrolide isolated from the sponge Chondrosia corticata, is associated with G2/M cell cycle arrest and suppression of mTOR signaling in human lung cancer cells. Toxicol In Vitro 27(2):694–699. https://doi.org/10.1016/j.tiv.2012.11.001
Liu J, Ma L, Wu N, Liu G, Zheng L, Lin X (2014) Aplysin sensitizes cancer cells to TRAIL by suppressing P38 MAPK/survivin pathway. Mar Drugs 12(9):5072–5088. https://doi.org/10.3390/md12095072
Gong AJ, Gong LL, Yao WC, Ge N, Lu LX, Liang H (2015) Aplysin induces apoptosis in glioma cells through HSP90/AKT pathwa. Exp Biol Med (Maywood, NJ) 240(5):639–644. https://doi.org/10.1177/1535370214555664
Pettit GR, Herald CL, Allen MS, von Dreele RB, Vanell LD, Kao JP, Blake W (1977) The isolation and structure of aplysistatin. J Am Chem Soc 99(1):262–263. https://doi.org/10.1021/ja00443a055
Hanusova V, Skalova L, Kralova V, Matouskova P (2015) Potential anti-cancer drugs commonly used for other indications. Curr Cancer Drug Targets 15(1):35–52
Whibley CE, McPhail KL, Keyzers RA, Maritz MF, Leaner VD, Birrer MJ, Davies-Coleman MT, Hendricks DT (2007) Reactive oxygen species mediated apoptosis of esophageal cancer cells induced by marine triprenyl toluquinones and toluhydroquinones. Mol Cancer Ther 6(9):2535–2543. https://doi.org/10.1158/1535-7163.mct-06-0760
Mun B, Wang W, Kim H, Hahn D, Yang I, Won DH, Kim EH, Lee J, Han C, Kim H, Ekins M, Nam SJ, Choi H, Kang H (2015) Cytotoxic 5alpha,8alpha-epidioxy sterols from the marine sponge Monanchora sp. Arch Pharm Res 38(1):18–25. https://doi.org/10.1007/s12272-014-0480-8
Diaz-Marrero A, Issi N, Canales V, Chamy C, San-Martin A, Darias J, Rovirosa J (2008) New diterpenes from the marine pulmonate Trimusculus peruvianus. Nat Prod Res 22(17):1516–1520. https://doi.org/10.1080/14786410701727812
van Wyk AW, Gray CA, Whibley CE, Osoniyi O, Hendricks DT, Caira MR, Davies-Coleman MT (2008) Bioactive metabolites from the South African marine mollusk Trimusculus costatus. J Nat Prod 71(3):420–425. https://doi.org/10.1021/np070612y
Salcedo M, Cuevas C, Alonso JL, Otero G, Faircloth G, Fernandez-Sousa JM, Avila J, Wandosell F (2007) The marine sphingolipid-derived compound ES 285 triggers an atypical cell death pathway. Apoptosis 12(2):395–409. https://doi.org/10.1007/s10495-006-0573-z
Nalini S, Sandy Richard D, Mohammed Riyaz SU, Kavitha G, Inbakandan D (2018) Antibacterial macro molecules from marine organisms. Int J Biol Macromol 115:696–710. https://doi.org/10.1016/j.ijbiomac.2018.04.110
Kareh M, El Nahas R, Al-Aaraj L, Al-Ghadban S, Naser Al Deen N, Saliba N, El-Sabban M, Talhouk R (2018) Anti-proliferative and anti-inflammatory activities of the sea cucumber Holothuria polii aqueous extract. 6:2050312118809541. https://doi.org/10.1177/2050312118809541
Carballo JL, Hernandez-Inda ZL, Perez P, Garcia-Gravalos MD (2002) A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol 2:17
Khademvatan S, Eskandari A, Saki J, Foroutan-Rad M (2016) Cytotoxic activity of Holothuria leucospilota extract against Leishmania infantum in vitro. Adv Pharmacol Sci 2016:8195381. https://doi.org/10.1155/2016/8195381
Dai J, Liu Y, Jia H, Zhou YD, Nagle DG (2007) Benzochromenones from the marine crinoid Comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J Nat Prod 70(9):1462–1466. https://doi.org/10.1021/np070224w
Janakiram NB, Mohammed A, Rao CV (2015) Sea cucumbers metabolites as potent anti-cancer agents. Mar Drugs 13(5):2909–2923. https://doi.org/10.3390/md13052909
Cuong NX, Vien LT, Hoang L, Hanh TTH, Thao DT, Thanh NV, Nam NH, Thung DC, Kiem PV, Minh CV (2017) Cytotoxic triterpene diglycosides from the sea cucumber Stichopus horrens. Bioorg Med Chem Lett 27(13):2939–2942. https://doi.org/10.1016/j.bmcl.2017.05.003
Silchenko AS, Avilov SA, Kalinin VI, Kalinovsky AI, Dmitrenok PS, Fedorov SN, Stepanov VG, Dong Z, Stonik VA (2008) Constituents of the sea cucumber Cucumaria okhotensis. Structures of okhotosides B1-B3 and cytotoxic activities of some glycosides from this species. J Nat Prod 71(3):351–356. https://doi.org/10.1021/np0705413
Li X, Roginsky AB, Ding XZ, Woodward C, Collin P, Newman RA, Bell RH Jr, Adrian TE (2008) Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann N Y Acad Sci 1138:181–198. https://doi.org/10.1196/annals.1414.025
Dyshlovoy SA, Madanchi R, Hauschild J, Otte K, Alsdorf WH, Schumacher U, Kalinin VI, Silchenko AS, Avilov SA, Honecker F, Stonik VA, Bokemeyer C, von Amsberg G (2017) The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophagy in urothelial carcinoma cells. BMC Cancer 17(1):93. https://doi.org/10.1186/s12885-017-3085-z
Sajwani FH, Collin P, Adrian TE (2017) Frondoside A potentiates the effects of conventional therapeutic agents in acute leukemia. Leukemia Res 63:98–108. https://doi.org/10.1016/j.leukres.2017.11.002
Zhao Q, Xue Y, Liu ZD, Li H, Wang JF, Li ZJ, Wang YM, Dong P, Xue CH (2010) Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J Food Sci 75(9):H280–H288. https://doi.org/10.1111/j.1750-3841.2010.01837.x
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs 9(10):1761–1805. https://doi.org/10.3390/md9101761
Sahara H, Hanashima S, Yamazaki T, Takahashi S, Sugawara F, Ohtani S, Ishikawa M, Mizushina Y, Ohta K, Shimozawa K, Gasa S, Jimbow K, Sakaguchi K, Sato N, Takahashi N (2002) Anti-tumor effect of chemically synthesized sulfolipids based on sea urchin’s natural sulfonoquinovosylmonoacylglycerols. Jpn J Cancer Res 93(1):85–92. https://doi.org/10.1111/j.1349-7006.2002.tb01204.x
Yang P, Collin P, Madden T, Chan D, Sweeney-Gotsch B, McConkey D, Newman RA (2003) Inhibition of proliferation of PC3 cells by the branched-chain fatty acid, 12-methyltetradecanoic acid, is associated with inhibition of 5-lipoxygenase. Prostate 55(4):281–291. https://doi.org/10.1002/pros.10243
Watjen W, Ebada SS, Bergermann A, Chovolou Y, Totzke F, Kubbutat MHG, Lin W, Proksch P (2017) Cytotoxic effects of the anthraquinone derivatives 1’-deoxyrhodoptilometrin and (S)-(-)-rhodoptilometrin isolated from the marine echinoderm Comanthus sp. Arch Toxicol 91(3):1485–1495. https://doi.org/10.1007/s00204-016-1787-7
Wright AD, Nielson JL, Tapiolas DM, Motti CA, Ovenden SP, Kearns PS, Liptrot CH (2009) Detailed NMR, including 1,1-ADEQUATE, and anticancer studies of compounds from the echinoderm Colobometra perspinosa. Mar Drugs 7(4):565–575. https://doi.org/10.3390/md7040565
Wang W, Hong J, Lee CO, Im KS, Choi JS, Jung JH (2004) Cytotoxic sterols and saponins from the starfish Certonardoa semiregularis. J Nat Prod 67(4):584–591. https://doi.org/10.1021/np030427u
Thao NP, Cuong NX, Luyen BT, Nam NH, Cuong PV, Thanh NV, Nhiem NX, Hanh TT, Kim EJ, Kang HK, Kiem PV, Minh CV, Kim YH (2013) Steroidal constituents from the starfish Astropecten polyacanthus and their anticancer effects. Chem Pharm Bull 61(10):1044–1051. https://doi.org/10.1248/cpb.c13-00490
Palanisamy SK, Trisciuoglio D, Zwergel C, Del Bufalo D, Mai A (2017) Metabolite profiling of ascidian Styela plicata using LC-MS with multivariate statistical analysis and their antitumor activity. J Enzyme Inhib Med Chem 32(1):614–623. https://doi.org/10.1080/14756366.2016.1266344
Russo GL, Ciarcia G, Presidente E, Siciliano RA, Tosti E (2008) Cytotoxic and apoptogenic activity of a methanolic extract from the marine invertebrate Ciona intestinalis on malignant cell lines. Med Chem (Shariqah (United Arab Emirates)) 4(2):106–109
Zheng LH, Wang YJ, Sheng J, Wang F, Zheng Y, Lin XK, Sun M (2011) Antitumor peptides from marine organisms. Mar Drugs 9(10):1840–1859. https://doi.org/10.3390/md9101840
Tardy C, Sabourdy F, Garcia V, Jalanko A, Therville N, Levade T, Andrieu-Abadie N (2009) Palmitoyl protein thioesterase 1 modulates tumor necrosis factor alpha-induced apoptosis. Biochim Biophys Acta 1793(7):1250–1258. https://doi.org/10.1016/j.bbamcr.2009.03.007
Rebecca VW, Nicastri MC, Fennelly C, Chude CI, Barber-Rotenberg JS, Ronghe A, McAfee Q, McLaughlin NP, Zhang G, Goldman AR, Ojha R, Piao S, Noguera-Ortega E (2019) PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discov 9(2):220–229. https://doi.org/10.1158/2159-8290.cd-18-0706
Lee S, LaCour TG, Fuchs PL (2009) Chemistry of trisdecacyclic pyrazine antineoplastics: the cephalostatins and ritterazines. Chem Rev 109(6):2275–2314. https://doi.org/10.1021/cr800365m
Vervoort H, Fenical W, Epifanio RA (2000) Tamandarins A and B: new cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J Org Chem 65(3):782–792. https://doi.org/10.1021/jo991425a
Andavan GS, Lemmens-Gruber R (2010) Cyclodepsipeptides from marine sponges: natural agents for drug research. Mar Drugs 8(3):810–834. https://doi.org/10.3390/md8030810
Broggini M, Marchini SV, Galliera E, Borsotti P, Taraboletti G, Erba E, Sironi M, Jimeno J, Faircloth GT, Giavazzi R, D’Incalci M (2003) Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia 17(1):52–59. https://doi.org/10.1038/sj.leu.2402788
Beesoo R, Neergheen-Bhujun V, Bhagooli R, Bahorun T (2014) Apoptosis inducing lead compounds isolated from marine organisms of potential relevance in cancer treatment. Mutat Res 768:84–97. https://doi.org/10.1016/j.mrfmmm.2014.03.005
Fayette J, Coquard IR, Alberti L, Boyle H, Meeus P, Decouvelaere AV, Thiesse P, Sunyach MP, Ranchere D, Blay JY (2006) ET-743: a novel agent with activity in soft-tissue sarcomas. Curr Opin Oncol 18(4):347–353. https://doi.org/10.1097/01.cco.0000228740.70379.3f
Clement JA, Kitagaki J, Yang Y, Saucedo CJ, O’Keefe BR, Weissman AM, McKee TC, McMahon JB (2008) Discovery of new pyridoacridine alkaloids from Lissoclinum cf. badium that inhibit the ubiquitin ligase activity of Hdm2 and stabilize p53. Bioorg Med Chem 16(23):10022–10028. https://doi.org/10.1016/j.bmc.2008.10.024
Tatsuta T, Hosono M, Rotinsulu H, Wewengkang DS, Sumilat DA, Namikoshi M, Yamazaki H (2017) Lissoclibadin 1, a polysulfur aromatic alkaloid from the Indonesian Ascidian Lissoclinum cf. badium, induces caspase-dependent apoptosis in human colon cancer cells and suppresses tumor growth in nude mice. J Nat Prod 80(2):499–502. https://doi.org/10.1021/acs.jnatprod.6b01051
Gandhi V, Plunkett W, Cortes JE (2014) Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res 20(7):1735–1740. https://doi.org/10.1158/1078-0432.ccr-13-1283
Yadav RR, Sharma S, Joshi P, Wani A, Vishwakarma RA, Kumar A, Bharate SB (2015) Meridianin derivatives as potent Dyrk1A inhibitors and neuroprotective agents. Bioorg Med Chem Lett 25(15):2948–2952. https://doi.org/10.1016/j.bmcl.2015.05.034
Bharate SB, Yadav RR, Battula S, Vishwakarma RA (2012) Meridianins: marine-derived potent kinase inhibitors. Mini Rev Med Chem 12(7):618–631. https://doi.org/10.2174/138955712800626728
Liberio MS, Sadowski MC, Davis RA, Rockstroh A, Vasireddy R, Lehman ML, Nelson CC (2015) The ascidian natural product eusynstyelamide B is a novel topoisomerase II poison that induces DNA damage and growth arrest in prostate and breast cancer cells. Oncotarget 6(41):43944–43963. https://doi.org/10.18632/oncotarget.6267
Won TH, Kim CK, Lee SH, Rho BJ, Lee SK, Oh DC, Oh KB, Shin J (2015) Amino acid-derived metabolites from the Ascidian Aplidium sp. Mar Drugs 13(6):3836–3848. https://doi.org/10.3390/md13063836
McHenry P, Wang WL, Devitt E, Kluesner N, Davisson VJ, McKee E, Schweitzer D, Helquist P, Tenniswood M (2010) Iejimalides A and B inhibit lysosomal vacuolar H+-ATPase (V-ATPase) activity and induce S-phase arrest and apoptosis in MCF-7 cells. J Cell Biochem 109(4):634–642. https://doi.org/10.1002/jcb.22438
Sikorska J, Hau AM, Anklin C, Parker-Nance S, Davies-Coleman MT, Ishmael JE, McPhail KL (2012) Mandelalides A-D, cytotoxic macrolides from a new Lissoclinum species of South African tunicate. J Org Chem 77(14):6066–6075. https://doi.org/10.1021/jo3008622
Nazari M, Serrill JD, Wan X, Nguyen MH (2017) New mandelalides expand a macrolide series of mitochondrial inhibitors. J Med Chem 60(18):7850–7862. https://doi.org/10.1021/acs.jmedchem.7b00990
Aiello A, Fattorusso E, Luciano P, Macho A, Menna M, Munoz E (2005) Antitumor effects of two novel naturally occurring terpene quinones isolated from the Mediterranean ascidian Aplidium conicum. J Med Chem 48(9):3410–3416. https://doi.org/10.1021/jm0489915
Acknowledgements
We convey our thanks to National Institute of Technology, Rourkela.
Funding
SP acknowledge DST-INSPIRE, Award reference number [IF180167], Department of Science and Technology, Government of India for providing fellowship. Research support was partly provided by Council of Scientific & Industrial Research (CSIR) [Grant Number: 37(1715)/18/EMR-II], Government of India.
Author information
Authors and Affiliations
Contributions
SP, PPP, DPP, BP, CSB and KKM have prepared the manuscript and table. SP, DPP, SRM and BPB have contributed towards the preparation of figure. SKB, MJ and SGP have done the proof reading of the manuscript. SKB, SKP and GS have conceptualized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
No human participants and/or animals have been used in this study.
Informed consent
The corresponding author on behalf of all the coauthors agree to accept the informed consent of compliance with ethical Standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 A and 1 B: Chemical structure of some potent anti-cancer compounds from the phylum Proifera with PubChem CID
:(1) Jaspamide (129,630,607), (2) Theopapuamide B (25,193,942), (3) Arenastatin A (15,221,174), (4) Hemiasterlin A (5,352,091), (5) Ciliatamides D (71,665,545), (6) Polytheonamide A (44,602,391), (7) Psammaplin A (6,400,741), (8) Halichondrin B (54,742,369), 9 A. Azumamide A (16,095,102), 9 B. Azumamide E (16,095,101), 10. Phakellistatin 14 (11,308,763), 11. Discorhabdin A (135,770,385), 12. Discodermolide (643,668), 13. Spongistatin 1 (9,963,674). (TIFF 1029 kb)
Supplementary Fig. 2: Chemical structure of some potent anti-cancer compounds from the phylum Cnidaria with PubChem CID
: 1 A. Klysimplexin B (44,243,824), 1 B. Klysimplexin H (44,243,827), 2. Crassocolide P (46,930,683), 3. Bielschowskysin (11,201,358), 4. Denticulatolide (10,430,489), 5. Cespitularin C (10,017,010), 6. Bromovulone I (5,283,227), 7. Capnell-9(12)-ene-8β,10α-diol (14,060,597), 8. Punaglandin I (5,283,242). (TIFF 781 kb)
Supplementary Fig. 3: Chemical structure of some potent anti-cancer compounds from the phylum Mollusca with PubChem CID
: 1 A. Dolastatin 3 (9,852,693), 1 B. Dolastatin 10 (9,810,929), 1 C. Dolastatin 11 (354,399), 1 D. Dolastatin 16 (177,386), 1 E. Dolastatin D (10,099,606), 2. Kulolide (10,417,850), 3. Aurilide B (11,721,984), 4. Dolabelide C (10,581,167), 5. Kahalalide F (6,436,220), 6. Kabiramide C (5,288,658), 7. Halichondramide (6,443,267), 8. Aplysin (11,066,347), 9. Aplysistatin (100,219), 10. Turbostatin 1 (11,456,614), 11. Spisulosines (9,925,886). (TIFF 618 kb)
Supplementary Fig. 4: Chemical structure of some potent anti-cancer compounds from the phylum Echinodermata with PubChem CID
:1) Stichoposide (119,095), (2) Frondoside A (23,664,994), (3) Cucumarioside A2 (23,665,000), (4) Holothurin A (44,559,168), (5) 12-methyltetradecanoic acid (12-MTA) (21,672), (6) Rhodoptilometrin (625,242). (TIFF 575 kb)
Supplementary Fig. 5: Chemical structure of some potent anti-cancer compounds from the phylum Ascadians and Tunicates with PubChem CID
: (1) Didemnin B (122,651), (2) Patellamide A (157,454), (3) Aplidine (9,812,534), (4) Trunkamide (9,811,230), (5) Ecteinascidin-743 (ET-743) (108,150), (6) Lissoclibadin 2 (11,657,934), (7) Lissoclinotoxin F (10,077,362), (8) Riitterazines (56,928,164), (9) Iejimalides A (5,473,233). (TIFF 787 kb)
Rights and permissions
About this article
Cite this article
Patra, S., Praharaj, P.P., Panigrahi, D.P. et al. Bioactive compounds from marine invertebrates as potent anticancer drugs: the possible pharmacophores modulating cell death pathways. Mol Biol Rep 47, 7209–7228 (2020). https://doi.org/10.1007/s11033-020-05709-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05709-8