Abstract
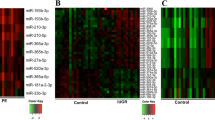
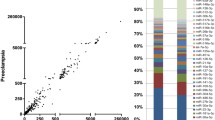
Preeclampsia (PE) and intrauterine growth restriction (IUGR) are pregnancy complications resulting from abnormal placental development. MicroRNAs can regulate placental development and contribute to disease, by influencing gene expression. Our previous study revealed an increase in miR-193b-5p expression in placentae from patients with early-onset pregnancy complications and identified candidate gene targets for miR-193b-5p. The purpose of this study is two-fold, first to validate candidate gene targets predicted for miR-193b-5p from microRNA-RNA expression data. Second, to overexpress miR-193b-5p in a trophoblast cell line (HTR-8/SVneo) to assess impact on trophoblast cell proliferation and migration. Integration of the miRNA and RNA sequencing expression data revealed 10 candidate gene targets for miR-193b-5p across all patient groups (PE only, IUGR only, PE + IUGR). Luciferase experiments identified two gene targets for miR-193b-5p, APLN and FGF13. Real-time PCR confirmed a median 45% decrease of FGF13 expression across 3 patient groups, and 50% decrease of APLN expression in patients with PE + IUGR. Following transfection of HTR-8/SVneo cells with miR-193b-5p mimics, APLN and FGF13 mRNA expression in HTR-8/SVneo was reduced by a median percentage of 30% and 45%, respectively. Concomitantly, HTR-8/SVneo cells demonstrate 40% reduction in cell migration. APLN and FGF13 immunoreactivity was identified strongly in the cytotrophoblast cells of the human placentae. These findings suggest that miR-193b-5p may contribute to trophoblast dysfunction observed in pregnancy complications such as PE and IUGR.





Similar content being viewed by others
Abbreviations
- APLN:
-
Apelin
- CT:
-
Cytotrophoblast
- EO:
-
Early-onset
- EVT:
-
Extravillous trophoblast
- FGF13:
-
Fibroblast growth factor 13
- GO:
-
Gene ontology
- IHC:
-
Immunohistochemistry
- IUGR:
-
Intrauterine growth restriction
- miRNA:
-
MicroRNA
- NGS:
-
Next generation sequencing
- PE:
-
Preeclampsia
- qRT-PCR:
-
Quantitative real time PCR
- SCT:
-
Syncytiotrophoblast
References
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402. https://doi.org/10.3389/fendo.2018.00402
Gebert LFR, MacRae IJ (2019) Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 20:21–37. https://doi.org/10.1038/s41580-018-0045-7
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597–610. https://doi.org/10.1038/nrg2843
Malnou EC, Umlauf D, Mouysset M, Cavaille J (2019) Imprinted microRNA gene clusters in the evolution, development, and functions of mammalian placenta. Front Genet 9:706. https://doi.org/10.3389/fgene.2018.00706
Mouillet JF, Ouyang Y, Coyne CB, Sadovsky Y (2015) MicroRNAs in placental health and disease. Am J Obstet Gynecol 213:S163–S172. https://doi.org/10.1016/j.ajog.2015.05.057
Phipps E, Thadhani R, Benzing T, Karumanchi SA (2019) Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 15:275–289. https://doi.org/10.1038/s41581-019-0119-6
American College of Obstetricians and Gynecologists (2019) ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol 133:e1–e25. https://doi.org/10.1097/AOG.0000000000003019
Myatt L (2002) Role of placenta in preeclampsia. Endocrine 19:103–111. https://doi.org/10.1385/ENDO:19:1:103
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN et al (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48:333–339. https://doi.org/10.1002/uog.15884
Roberts DJ, Post MD (2008) The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol 61:1254–1260. https://doi.org/10.1136/jcp.2008.055236
Miloseic-Stevanovic J, Krstic M, Radovic-Janosevic D, Stefanovic M, Antic V, Djordjevic I (2016) Preeclampsia with and without intrauterine growth restriction–Two pathogenetically different entities? Hypertens Pregnancy 35:573–582. https://doi.org/10.1080/10641955.2016.1212872
Schoots MH, Gordijn SJ, Scherjon SA, Van Goor H, Hillebrands JL (2018) Oxidative stress in placental pathology. Placenta 69:153–161. https://doi.org/10.1016/j.placenta.2018.03.003
Awamleh Z, Gloor GG, Han VKM (2019) Placental microRNAs in pregnancies with early onset intrauterine growth restriction and preeclampsia: potential impact on gene expression and pathophysiology. BMC Med Genomics 12:91–101. https://doi.org/10.1186/s12920-019-0548-x
Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T et al (2012) Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: A novel marker for predicting preeclampsia. Hypertension 59:265–273. https://doi.org/10.1161/HYPERTENSIONAHA.111.180232
Betoni JS, Derr K, Pahl MC, Rogers L, Muller CL, Packard RE et al (2013) MicroRNA analysis in placentas from patients with preeclampsia: Comparison of new and published results. Hypertens Pregnancy 32:321–339. https://doi.org/10.3109/10641955.2013.807819
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li YX et al (2014) Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 63:1276–1284. https://doi.org/10.1161/HYPERTENSIONAHA.113.02647
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. https://doi.org/10.1038/nprot.2007.30
Zhou X, Li Q, Xu J, Zhang X, Zhang H, Xiang Y et al (2016) The aberrantly expressed miR-193b-3p contributes to preeclampsia through regulating transforming growth factor-β signaling. Sci Rep 6:19910. https://doi.org/10.1038/srep19910
Forbes K, Souquet B, Garside R, Aplin JD, Westwood M (2010) Transforming growth factor-{beta} (TGF{beta}) receptors I/II differentially regulate TGF{beta}1 and IGF-binding protein-3 mitogenic effects in the human placenta. Endocrinology 151:1723–1731. https://doi.org/10.1210/en.2009-0896
Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T et al (2008) Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J 27:522–534. https://doi.org/10.1038/sj.emboj.7601982
Japp AG, Newby DE (2008) The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol 75:1882–1892. https://doi.org/10.1016/j.bcp.2007.12.015
Furuya M, Okuda M, Usui H, Takenouchi T, Kami D, Nozawa A et al (2012) Expression of angiotensin II receptor-like 1 in the placentas of pregnancy-induced hypertension. Int J Gynecol Pathol 31:227–235. https://doi.org/10.1097/PGP.0b013e31823b6e71
Inuzuka H, Nishizawa H, Inagaki A, Suzuki M, Ota S, Miyamura H et al (2013) Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy 32:410–421. https://doi.org/10.3109/10641955.2013.813535
Yamaleyeva LM, Chappell MC, Brosnihan KB, Anton L, Caudell DL, Shi S et al (2015) Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab 309:E852–E860. https://doi.org/10.1152/ajpendo.00272.2015
Van Mieghem T, Doherty A, Baczyk D, Drewlo S, Baud D, Carvalho J, Kingdom J (2016) Apelin in normal pregnancy and pregnancies complicated by placental insufficiency. Reproduct Sci 23:1037–1043. https://doi.org/10.1177/1933719116630422
Nishizawa H, Ota S, Suzuki M, Takema K, Sekiya T, Kurahashi H, Udagawa Y (2011) Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol 9:107–119. https://doi.org/10.1186/1477-7827-9-107
Mayeur A, Wattez JS, Lukaszewski MA, Lecoutre S, Butruille L, Drougard A et al (2016) Apelin control fetal and neonatal glucose homeostasis and is altered by maternal undernutrition. Diabetes 65:554–560. https://doi.org/10.2337/db15-0228
Vaughan OE, Powell TL, Jansson T (2019) Apelin is a novel regulator of human trophoblast amino acid transport. Am J Physiol Endocrinol Metab 316:E810–E816. https://doi.org/10.1152/ajpendo.00012.2019
Goldfarb M (2005) Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev 16:215–220. https://doi.org/10.1016/j.cytogfr.2005.02.002
Wu QF, Yang L, Li S, Wang Q, Yuan XB, Gao X et al (2012) Fibroblast growth factor 13 is a microtubule-stabilizing protein regulating neuronal polarization and migration. Cell 149:1549–1564. https://doi.org/10.1016/j.cell.2012.04.046
Lu H, Shi X, Wu G, Zhu J, Song C, Zhang Q, Yang G (2015) FGF13 regulates proliferation and differentiation of skeletal muscle by down-regulating Spry1. Cell Prolif 48:550–560. https://doi.org/10.1111/cpr.12200
Bublik DR, Bursać S, Sheffer M, Orsolic I, Shalit T, Tarcic O et al (2017) Regulatory module involving FGF13, miR-504, and p53 regulates ribosomal biogenesis and supports cancer cell survival. Proc Natl Acad Sci USA 114:E496–E505. https://doi.org/10.1073/pnas.1614876114
Nishimoto S, Nishida E (2007) Fibroblast growth factor 13 is essential for neural differentiation in Xenopus early embryonic development. J Biol Chem 282:24255–24261. https://doi.org/10.1074/jbc.M704277200
Yue X, Sun Y, Zhong M, Ma Y, Wei Y, Sun F et al (2018) Decreased expression of fibroblast growth factor 13 in early-onset preeclampsia is associated with the increased trophoblast permeability. Placenta 62:43–49. https://doi.org/10.1016/j.placenta.2017.12.009
Jin HY, Gonzalez-Martin A, Miletic AV, Lai M, Knight S, Sabouri-Ghomi M et al (2015) Transfection of microRNA mimics should be used with caution. Front Genet 6:340. https://doi.org/10.3389/fgene.2015.00340
Goldgraben MA, Russell R, Rueda OM, Caldas C, Git A (2016) Double-stranded microRNA mimics can induce length- and passenger strand-dependent effects in a cell-type specific manner. RNA 22:193–203. https://doi.org/10.1261/rna.054072.115
Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM et al (2007) Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196:1–6. https://doi.org/10.1016/j.ajog.2007.01.008
Gastaldi C, Bertero T, Xu N, Bourget-Ponzio I, Lebrigand K, Fourre S et al (2013) miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis 35:1110–1120. https://doi.org/10.1093/carcin/bgt490
Khordadmehr M, Shahbazi R, Sadreddini S, Baradaran B (2019) miR-193b: a new weapon against cancer. J Cell Physiol 234:16861–16872. https://doi.org/10.1002/jcp.28368
Blick C, Ramachandran A, McCormick R, Wigfield S, Cranston D, Catto J, Harris AL (2015) Identification of a hypoxia-regulated miRNA signature in bladder cancer and a role for miR-145 in hypoxia-dependent apoptosis. Br J Cancer 113:634–644. https://doi.org/10.1038/bjc.2015.203
Hu H, Li S, Liu J, Ni B (2012) MicroRNA-193b modulates proliferation, migration, and invasion of non-small cell lung cancer cells. Acta Biochim Biophys Sin 44:424–430. https://doi.org/10.1093/abbs/gms018
Hulin JA, Tommasi S, Elliot D, Hu DG, Lewis BC, Mangoni AA (2017) MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase 1. Sci Rep 7:1–15. https://doi.org/10.1038/s41598-017-14454-1
Shin CH, Lee H, Kim HR, Choi KH, Joung JG, Kim HH (2017) Regulation of PLK1 through competition between hnRNPK, miR-149-3p and miR-193b-5p. Cell Death Differ 24:1861–1871. https://doi.org/10.1038/cdd.2017.106
Lyall F, Robson SC, Bulmer JN (2013) Spiral artery remodelling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62:1046–1054. https://doi.org/10.1161/HYPERTENSIONAHA.113.01892
Desforges M, Harris LK, Aplin JD (2015) Elastin-derived peptides stimulate trophoblast migration and invasion: a positive feedback loop to enhance spiral artery remodelling. Mol Hum Reprod 21:95–104. https://doi.org/10.1093/molehr/gau089
Vidal DO, Ramao A, Pinheiro DG, Muys BR, Lorenzi JCC, de Padua AC et al (2018) Highly expressed placental miRNAs control key biological processes in human cancer cell lines. Oncotarget 9:23554–23563. https://doi.org/10.18632/oncotarget.25264
Acknowledgements
We would like to thank all the donors and the Research Centre for Women’s and Infants Health (RCWIH) BioBank for placental samples used in this project. We would also like to acknowledge Karen Nygard (Biotron Facility, Western University) for assistance with immunohistochemical staining of placental tissues.
Funding
This study was funded by grants from the Canadian Institutes of Health Research (15579 and 15262 to VKMH) and The Douglas and Vivian Bocking Chair in Fetal and Newborn Growth (to VKMH). ZA is supported through Western University’s Graduate Research Scholarship and the Graduate Student Grant from Western University’s Department of Paediatrics.
Author information
Authors and Affiliations
Contributions
ZA made substantial contributions to design, acquisition of data, analysis and interpretation of data, and in writing and revising the article. VKMH made substantial contributions to design, interpretation of data and revising the article. All authors approved final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The author have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Negative control images for immunohistochemical analysis. Slides designated negative controls underwent the same procedures, with the exception of the application of the primary antibody. (A) Chorionic villus section and (B) Basal plate decidua section from preterm control, gestational age 29+4. Blue staining is CAT Hematoxylin counterstain. All images were captured at 20 x, bar = 50 m. (TIFF 43115 kb)
Rights and permissions
About this article
Cite this article
Awamleh, Z., Han, V.K.M. Potential pathophysiological role of microRNA 193b-5p in human placentae from pregnancies complicated by preeclampsia and intrauterine growth restriction. Mol Biol Rep 47, 6531–6544 (2020). https://doi.org/10.1007/s11033-020-05705-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05705-y